Case Report
When a STEMI is Just the Tip of an Iceberg: An Acute Coronary Syndrome Revealing an Essential Thrombocythemia
1. Cardiology Department, Blida University Hospital, Algeria.
2. Cardiology Department, Issaad Hassani Algiers University Hospital, Algeria.
*Corresponding Author: M.A Bouraghda, Cardiology Department, Blida University Hospital, Algeria.
Citation: M.A Bouraghda, S Zighoud, H Gasmi, M-A Bouzid (2024). When a STEMI is Just the Tip of an Iceberg: An Acute Coronary Syndrome Revealing an Essential Thrombocythemia. Journal of Clinical Cardiology and Cardiology Research, BioRes Scientia Publishers. 3(1):1-4. DOI: 10.59657/2837-4673.brs.24.025.
Copyright: © 2024 M.A Bouraghda, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: February 14, 2024 | Accepted: March 11, 2024 | Published: March 16, 2024
Abstract
Essential thrombocythemia (ET) is a myeloproliferative disorder characterized by monoclonal proliferation of hematopoietic stem cells with persistent high platelet counts. It can remain asymptomatic, it is generally discovered incidentally, but it can also manifest itself by thrombotic complications, which are more frequent and often more severe than hemorrhagic manifestations and can also be a mode of disease discovery and enable diagnosis, such is the case of our patient.
Keywords: essential thrombocythemia; acute coronary syndrome
Introduction: Case report
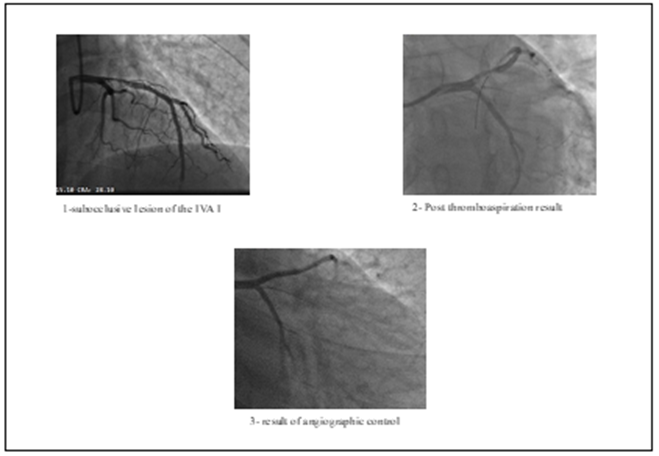
Mr. TM 46 years old without modifiable cardiovascular risk factors, with a history of an undocumented acute coronary syndrome at the age of 30, without an etiological exploration. He consults us for acute chest pain that started 7 hours ago. The patient is conscious and cooperating with good mucocutaneous coloring, without obvious hemorrhagic syndrome, eupneic at rest and with a persistent chest pain. The cardiovascular examination is normal, BP: 130/70 mm Hg in both superior limbs. The ECG demonstrates an anterior STEMI with the appearance of sequelae of necrosis in the correspondent territory. Biology finds a thrombocytosis 850 000 units /mm3, the rest of the parameters are normal. In view of the clinical and electrical data, an emergency coronary angiography was performed revealing a sub-occlusive and thrombotic lesion of the proximal left anterior descending artery (LAD) with TIMI flow at 2. Thromboaspiration was done restoring a 3 TIMI flow with persistence of a heterogeneous appearance, for which we indicated the continuous administration of anti GPIIbIIIa for 24 hours.
Figure 1
The hospital checkup was simple, transthoracic echocardiography revealed a non-dilated, non-hypertrophied LV, severe hypokinesia of the anterior and anteroseptal walls with a mid-range LVEF at 40%, non-elevated filling pressures, no Pulmonary hypertension, no significant valvular defects, and pericardial effusion. The post-coronary angiogram renal assessment was correct, the radial puncture site was free of hematoma with a preserved radial pulse, without signs of limb ischemia. The initiation of the exploration of thrombocytosis in hematology revealed giant platelets and megakaryocytic fragments in the blood smear. A bone marrow puncture was done to rule out any reactive thrombocytosis, particularly in myelodysplastic syndromes and myeloproliferative neoplasia.
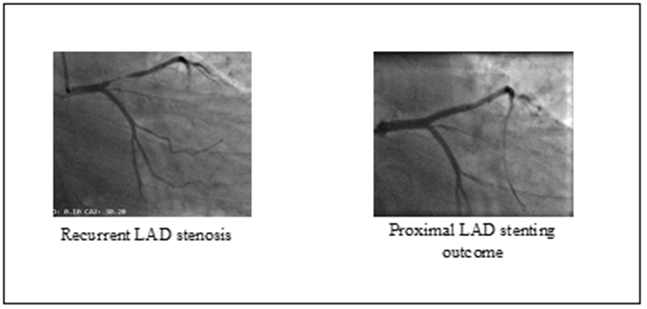
The genetic study revealed a mutation of the JAK2 V617F gene. Faced with this set of arguments, the diagnosis of essential thrombocythemia was established and the patient was placed on hydroxyurea at a dose of 500 mg twice/day, in addition to a double anti-platelet therapy as aspirin 100 mg/day-clopidogrel 75 mg as well, as the rest of the treatment for ischemic heart disease. On the angiographic control after one week, we found a complete cleaning of the thrombotic lesion on the proximal LAD. The patient is, therefore, outgoing with regular follow-up appointments. After 3 months, the patient reported typical stress angina stage 2 of the CCS classification, the ECG showed an aspect of semi-recent STEMI, the transthoracic echocardiography did not show any new kinetic abnormality. Furthermore, a control blood count finds the platelets amount at 400 000/mm3. Given the symptoms, a myocardial scintigraphy is done revealing the effects of necrosis at the apical portion of the LV (3 segments/17) as well as moderate to severe stress ischemia that is almost reversible in the LAD territory. estimated at 5/17 segments. Hence the indication for coronary angiography, which demonstrates a very tight stenosis at the same level of the previous lesion. We opted for a direct stenting with a 15*3.5mm drug eluting stent, which was done successfully.
Figure 2
There were no subsequent complications, the patient was discharged with prolonged double anti-aggregation. Controls at 3-6-12 months showed no ischemic or hemorrhagic events, the patient is totally asymptomatic and platelet count was normal.
Discussion
Essential thrombocythemia is a chronic myeloproliferative syndrome characterized by a sustained elevation of platelet count exceeding 450 000/mm3 [1,3]. It is a rare disease, occurring in 1 to 2.5 cases per 100 000 inhabitants annually in France 2. It most commonly occurs after the age of 50, affecting both men and women, with a frequency peak around 30 years, especially in women [2]. Although the life expectancy of patients with this syndrome is not significantly altered, morbidity and mortality related to vascular, hemorrhagic, or thrombotic complications are notable in this population 3. Thromboembolic complications are observed in almost half of the patients at the time of diagnosis (20-50%) [4,5,6,] and hemorrhagic complications occur in 16 to 20% [6]. These vascular complications result from qualitative and quantitative platelet defects [7] but seem to be associated more with qualitative abnormalities than quantitative ones [8]. Myocardial infarction is a rare complication, with a prevalence of 9.4% 9. The main mechanisms implicated are abnormalities in the activation of the fibrinolytic system, reinforcement of platelet procoagulant activity, and increased blood viscosity. The diagnosis of essential thrombocythemia is made after excluding reactive thrombocytosis (inflammatory syndrome, iron deficiency, or asplenia) and other myeloproliferative syndromes [10]. The main molecular marker of myeloproliferative syndromes in the absence of the Philadelphia chromosome is the JAK2 V617F gene mutation, present in half of essential thrombocythemia [10]. Several factors have been associated with an increased risk of thromboembolic complications of essential thrombocythemia and have been included in an "IPSET thrombosis" score (International Prognostic Score for Essential Thrombocythemia). Factors included in this score are age over 60 years, history of thrombotic events, cardiovascular risk factors, and the JAK2 V617F mutation [11] (Table 1).
The risk is stratified into three categories: low (total score = 0–1), intermediate (total score = 2), and high (total score = 3).
Table 1: "IPSET thrombosis" score (International Prognostic Score for Essential Thrombocythemia).
| Risk Factor | HR | Score |
| Age >60 Years | 1.50 | 1 |
| Cardiovascular risk factors | 1.56 | 1 |
| Previous thrombosis | 1.93 | 2 |
| JAK2V617F | 2.04 | 2 |
Low risk implies a score = 0-1; intermediate risk, score = 2; and high risk, score greater than or equal to 3.
In our observation, the diagnosis of TE was suspected following coronary thrombosis with a high platelet count and confirmed subsequently by genetic study with the JAK2 V617F gene mutation. This mode of discovering the pathology has been associated with thrombotic complications [12].
The management of TE is currently based on practice recommendations 7:
- Young patients (age < 60>
- Patients with a history of thrombosis, hemorrhagic complications, platelet count > 1500000/mm3, or age > 60 years (high risk of thrombosis): hydroxyurea is the treatment of choice due to its effectiveness in preventing thrombotic complications and the rarity of acute toxicities [5,13]. The presence of a JAK2 V617F mutation is associated with better outcomes.
In the context of TE complicated by coronary thrombosis, inhibition of platelet aggregation and platelet production seems to play an important role in treatment (reducing recurrence of thrombotic events by up to 70%) [8,14].
Take home messages
- The search for an etiology of coronary events in young subjects is crucial.
- Essential thrombocytosis causes ischemic and hemorrhagic events, hence the need to pay attention to the hemorrhagic risk accentuated by dual anti-platelet therapy.
- Hydroxyurea decreases the rate of recurrence of ischemic events by reducing the number of platelets, hence the importance of its initiation after a coronary event when the thrombotic risk is high.
- The discovery of essential thrombocythemia following a thrombotic event carries a high risk of recurrence.
- The mutation of the JAK2V617F gene predicts a higher thrombotic risk.
References
- Giraudet G, Wibaut B, Ducloy A-S, et al. (2011). Thrombocytémie essentielle et grossesse. Gynecol Obstet Fertil. 39(4):205–210.
Publisher | Google Scholor - Rüfera A, Toblerb A, Tichellic A, et al. (2003). Syndromes myéloprolifératifs: polycythémie vraie, thrombocytémie essentielle, ostéomyélofibrose. Forum Med Suisse. 3(43):1026–1033.
Publisher | Google Scholor - Beer PA, Green AR. (2009). Pathogenesis and management of essential thrombocythemia. Hematology Am Soc Hematol Educ Program. (1):621–628.
Publisher | Google Scholor - Cortelazzo S, Finazzi G, Ruggeri M, et al. (1995). Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med. 332(17):1132–113
Publisher | Google Scholor - Wehmeir A, Daum I, Jamin H, et al. (1991). Incidence and clinical risk factors for bleeding and thrombotic complications in myeloproliferative disorder: Retrospective analysis of 260 patients. Ann hematol. 63(2):101–106.
Publisher | Google Scholor - Naoko Kumagai, Ryoko Mitsutake, Shin-ichiro Miura, Akira KawamuraYosuke Takamiya, Hiroaki Nishikawa, et al. (2009). Acute coronary syndrome associated with essential thrombocythemia.J Cardiol. 54(3):485-9. doi: 10.1016/j.jjcc.2009.03.001
Publisher | Google Scholor - E. Lagha, R. Tlili, F. Azaiez, R. Hentati, R. Ben Romdhane, et al. (2020). Infarctus du myocarde comme première manifestation clinique de la thrombocytémie essentielle. Revue Tunisienne de Cardiologie. Vol 16 N°4- 4e Trimestre.
Publisher | Google Scholor - Rossi C, Randi ML, Zerbinati P, et al. (1998). Coronary disease in essential thrombocythemia and polycythemia vera. J Intern Med. 244(1):49–53.
Publisher | Google Scholor - J. Kologo, A. Samadoulougou, G. Millogo, L. Kagambèga, P. Zabsonré. (2014). Infarctus du myocarde révélateur d’une thrombocytémie essentielle chez un sujet jeune noir africain : à propos d’une observation. PanAfrican Medical Journal.
Publisher | Google Scholor - Barbui T, Finazzi G, Carobbio A, Thiele J, Passamonti F, et al. (2012). Development and validation of an International Prognostic Score of thrombosis in World Health Organization–essential thrombocythemia (IPSET-thrombosis). Blood. 120(26):5128–33
Publisher | Google Scholor - Tortorella, G., Calzolari, M., Tieghi, A., Muià, N., Piccin, A., & Gugliotta, L. (2016). Acute coronary syndrome (ACS) in patients with essential thrombocytemia (ET).What is the best treatment? International Journal of Cardiology, 203, 225–227.
Publisher | Google Scholor - Koh KK, Cho SK, Kin SS, et al. (1993). Coronary vasospasm, multiple coronary thrombosis, unstable angina and essential thrombocytosis. Int J Cardiol. 41(2):168–170.
Publisher | Google Scholor - Anand Singla, Dinesh Jagasia, Mukesh Garg, Philip A Lowry, Dwight Stapleton. (2012). Acute ST-segment elevation myocardial infarction: a rare initial presentation of previously undiagnosed essential thrombocythemia. Platelets. 23(6):463-6.
Publisher | Google Scholor