Research Article
Using of Autologous PRP in Human Seminal Fluid Cryopreservation Technique
- Rana R Al-Saadi *
- Ula Al-Kawaz
- Nihal A. Kamal
High Institute of Infertility Diagnoses and ART, Al-Nahrain University, Iraq.
*Corresponding Author: Rana R Al-Saadi, High Institute of Infertility Diagnoses and ART, Al-Nahrain University, Iraq.
Citation: Al-Saadi R R, Al-Kawaz U, Kamal N A. (2024). Using of Autologous PRP in Human Seminal Fluid Cryopreservation Technique. Journal of Women Health Care and Gynecology, BioRes Scientia Publishers. 4(3):1-4. DOI: 10.59657/2993-0871.brs.24.067
Copyright: © 2024 Rana R Al-Saadi, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: November 11, 2024 | Accepted: December 04, 2024 | Published: December 10, 2024
Abstract
Background: Cryopreservation of seminal fluid is a technique used to preserve and store sperm cells at extremely-low temperatures, typically in liquid nitrogen at around -196 degrees Celsius. Autologous PRP therapy is using in assisted reproductive technologies (ART) due to its well-known regenerative potential.
Objective: to investigate the effect of using autologous platelet rich plasma as a cryoprotective media on human spermatozoa.
Study design: Sixty semen sample from normozoospermia male with their autologous PRP prepared from their blood samples were collected at the same time of semen collection and all semen samples were tested for macroscopic and microscopic examination. Each sample divided into three groups (Control sample only cryomedia glycerol, PRP sample 0.5 ml, and mixed sample 0.5ml PRP + Cryomedia glycerol). Then preserve these three groups into liquid nitrogen -196 for two months. After that, thawing in water bath at 37 C. then semen parameters were assessment and the results of the three groups were compared.
Results: The sixty semen samples that preserved with cryopreservation media only, showed significantly lower sperm parameters, while the samples that preserved with 0.5 ml PRP proved non-significant results.
Conclusion: Autologous PRP has negative effect on sperm parameters when used alone as a cryoprotective agent for semen cryopreservation.
Keywords: cryopreservation; cryoprotectant agent; autologous PRP; freez-thaw; normozoospermic; progressive motility
Introduction
Cryopreservation of semen includes the freezing of sperm cells at extremely low temperatures, in most cases around -196°C using liquid nitrogen. This process is essential for preserving the viability of sperm for prolonged periods, ensuring their availability for various reproductive applications [1]. There are several indications for using semen cryopreservation technique, such as assisted reproductive technologies in case of (IVF) in vitro fertilizition or (ICSI) intra cytoplasmic sperm injection [2]. Also Before Vasectomy or Sterilization[3]. The process involves three types which are slow freezing; rapid freezing or vitrification, each one requires carefully controlled freezing and storage in liquid nitrogen to maintain sperm viability, by optimization of cryopreservation protocols , cryoprotectant concentrations and freezing rates [4, 5]. There are two types of cryoprotectant agent as penetrating which include glycerol and ( DMSO ) dimethyl sulfoxide [6], and the second type is non penetrating which include sugars and polyvinylpyrrolidone (PVP) [7].
Platelet-rich plasma (PRP) is a component of blood containing a greater concentration of platelets than what is typically found in circulating blood. Platelets are pivotal for clotting and wound healing, and PRP is thought to encorage tissue repair and regeneration due to release various growth factors and cytokines, including platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), and vascular endothelial growth factor (VEGF) [8]. PRP has been used in numerous medical fields, including orthopedics, dermatology, and dentistry, due to its regenerative properties [9]. PRP’s success can be assigned to its ability to stimulate tissue repair and regeneration through the activation of several signaling pathways, including the recruitment of mesenchymal stem cells (MSCs). These cells possess multi-potent differentiation capabilities and can contribute to tissue repair and regeneration [10]. Platelet-rich plasma (PRP) has earned attention for its potential applications in various medical fields, including reproductive medicine in vitro fertilization (IVF) the impact of PRP on sperm parameters and fertility outcomes might have a positive effect on sperm motility and morphology to improved fertility outcomes [11]. PRP has gained attention as a potential cryoprotective agent due to its rich content of growth factors, proteins, and cytokines that could theoretically support cell survival during cryopreservation. However, the cytotoxic effects of PRP, specifically when used in high concentrations or under specific conditions, need to be thoroughly evaluated [12].
Materials and method
Sixty semen sample from normozoospermic male with their autologous PRP prepared from their blood samples were collected at the same time of semen collection and all semen samples were tested for macroscopic and microscopic examination. Each sample divided into three groups (Control sample only cryomedia glycerol 0.7 ml , PRP sample 0.5 ml , and mixed sample 0.5ml PRP+Cryomedia glycerol 0.7 ml ). Then preserve these three groups into liquid nitrogen -196 for two months . After that, thawing in water bath at 37 C. then semen parameters were evaluated and the results of the three groups were compared.
Statistical analysis
The data were analyzed using Statistical Package for Social Sciences (SPSS) version 23.0 and Microsoft office 2010.The descriptive statistics including frequency, range, mean and standard error were measured to describe the data .The groups were compared by applying paired sample t-test and analysis of variance. Post hoc tukey test of ANOVA were used to assess paired samples significance and the results were considered statistically significant when p value was equal to or less than 0.05.
Results
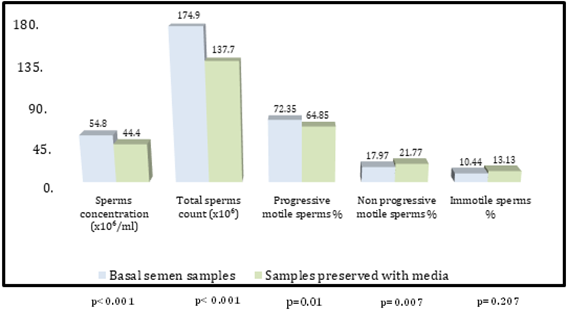
Comparison of seminal analysis parameters between basal samples and samples preserved with media. The comparison between basal semen samples and sample preserved with media were presented in table 1 and figure 1, accordingly the semen sample preserved with media showed significantly lower sperms concentration (44.40 ± 2.08 vs.54.80 ± 2.27; p less than 0.001), total sperms count (137.78 ± 8.92 vs 174.93 ± 8.79; p less than 0.001) and progressively motile sperms percent (64.85 ± 1.91vs.72.35 ± 2.61; p=0.010). There was also significantly higher non-progressively motile sperms percent (21.77 ± 1.39 vs. 17.97 ± 1.35; p=0.007) and insignificantly higher immotile sperms percent (13.13 ± 1.25 vs. 10.44 ± 1.75; p=0.207).
Table 1: Comparison of seminal analysis parameters between basal samples and samples preserved with media
| Parameters | Basal semen samples | Samples preserved with media | p value |
| Sperms concentration | 54.80 ± 2.27 | 44.40 ± 2.08 | less than 0.001 Ŧ |
| Total sperms count (106) | 174.93 ± 8.79 | 137.78 ± 8.92 | less than 0.001 Ŧ |
| Progressive motile sperms | 72.35 ± 2.61 | 64.85 ± 1.91 | 0.010 Ŧ S |
| Non progressive | 17.97 ± 1.35 | 21.77 ± 1.39 | 0.007 Ŧ S |
| Immotile sperm % | 10.44 ± 1.75 | 13.13 ± 1.25 | 0.207 Ŧ NS |
Ŧ: Paired sample t test; S: Significant (p ≤ 0.05); NS: Not significant (p > 0.05)
Figure 1: Comparison of seminal analysis parameters between basal samples and samples preserved with media
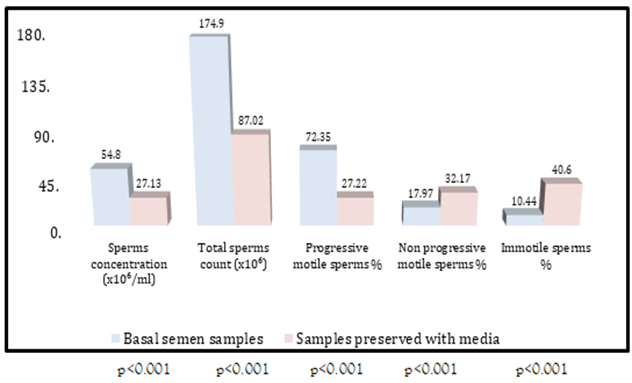
Comparison of seminal analysis parameters between basal samples and samples preserved with 0.5 ml PRP Samples preserved with 0.5 ml PRP also had significantly lower sperms concentration (27.13 ± 2.04 vs. 54.80 ± 2.27; p less than 0.001), total sperms count (87.02 ± 7.36 vs. 174.93 ± 8.79; p less than 0.001) and progressively motile sperms percent (27.22 ± 2.54 vs.72.35 ± 2.61; p less than 0.001) On the other hand there were significantly higher non-progressively motile sperms percent (32.17 ± 3.27 vs. 17.97 ± 1.35; p < 0>p less than 0.001) as demonstrated in table 2 and figure 2.
Table 2: Comparison of seminal analysis parameters between basal samples and samples preserved with 0.5 ml PRP
| Parameters | Basal semen samples | Samples preserved with 0.5 ml PRP | p value |
| Sperms concentration (106/ml) | 54.80 ± 2.27 | 27.13 ± 2.04 | less than 0.001 Ŧ S |
| Total sperms count (106) | 174.93 ± 8.79 | 87.02 ± 7.36 | less than 0.001 Ŧ S |
| Progressive motile sperms % | 72.35 ± 2.61 | 27.22 ± 2.54 | less than 0.001 Ŧ S |
| Non progressive motile sperms % | 17.97 ± 1.35 | 32.17 ± 3.27 | less than 0.001 Ŧ S |
| Immotile sperm % | 10.44 ± 1.75 | 40.60 ± 3.87 | less than 0.001 Ŧ S |
Ŧ: Paired sample t test; S: Significant (p ≤ 0.05); NS: Not significant (p > 0.05)
Figure 2: Comparison of seminal analysis parameters between basal samples and samples preserved with 0.5 ml PRP
Discussion
According to the comparison between the sperm parameters before the cryopreservation and post thawing in table1 and figure1 that preveals there were significantly lowering in the concentration , sperm count and progressive motile sperm percent, while there were also slightly increase in the non-progressive motile percent and immotile sperm showed no significant change , the findings suggest that the preservation of semen samples with media may adversely affect sperm parameters, which attributable to the effect of freezing on sperm cells. One of the primary affects with freezing and thawing sperm is the possible decline in motility and viability [13]. This decrease can be attributed to the formation of ice crystals, which may damage the sperm cell membrane [14]. These results underscore the importance of considering the impact of preservation methods on sperm quality. Samples preserved with 0.5 ml Platelet-Rich Plasma (PRP) exhibited striking differences compared to semen samples before cryopreservation, as depicted in Table 2 and Figure 2. These preserved samples displayed significantly lower sperm concentration, total sperm count, and progressively motile sperm percentage. Conversely, there was a notable increase in the percentage of non-progressively motile sperm and immotile sperm in samples preserved with 0.5 ml PRP. These findings highlight the detrimental effect of PRP preservation on sperm quality parameters. PRP, although touted for its regenerative properties, appears to negatively impact sperm parameters when used as a cryoprotective agent alone without cryoprotectant media for preservation. The substantial decrease in sperm concentration and motility, coupled with the significant increase in non-progressively motile and immotile sperm, underscores the need for caution when considering PRP as a preservation medium for semen samples. These results have implications for clinical and research practices in assisted reproductive technologies, emphasizing the importance of selecting preservation methods that maintain or enhance sperm quality to optimize reproductive outcomes.
Conclusion
In summary, due to the results of this study on the semen parameters, these conclusions were made, the addition of cryopreservation media only to the semen shows low positive results, while the addition of Autologous PRP 0.5 ml as a cryoprotective agent shows negative results.
References
- Purdy, P. H. (2006). A review on goat sperm cryopreservation. Small Ruminant Research, 63(3):215-225.
Publisher | Google Scholor - Esteves, S.C., Miyaoka, R., and Agarwal, A. (2015). An update on the clinical assessment of the infertile male. The World Journal of Men’s Health, 33(1):1-20.
Publisher | Google Scholor - World Health Organization. (2012). Vasectomy: A technical and policy review. Contraception, 85(5):432-444.
Publisher | Google Scholor - Smith, A. B., Stacey, G. N., & Reddi, P. P. (2019). Principles of cryopreservation applied to reproductive cells. In Principles of Regenerative Medicine (Third Edition). Academic Press, 1083-1100.
Publisher | Google Scholor - Jones, C., Kaya, A., Hu, J., Aitken, R. J., & Nixon, B. (2020). Optimization of cryopreservation protocols for spermatozoa. In Cryopreservation and Freeze-Drying Protocols Springer, 27-45.
Publisher | Google Scholor - Budiyanto, A., Abdullah, M. S., & Inggriani, C. (2018). Dimethyl sulfoxide (DMSO) as a Cryoprotectant for Chicken Semen: A Review. IOP Conference Series: Earth and Environmental Science, 130(1):012016.
Publisher | Google Scholor - Leibo, S. P. (2009). Osmotic and Cryoprotectant Permeability Characteristics of Mammalian Oocytes and Embryos. Biology of Reproduction, 81(1):135-146.
Publisher | Google Scholor - Molloy T, Wang Y, Murrell G. (2019). The roles of growth factors in tendon and ligament healing. Sports Medicine.
Publisher | Google Scholor - Nagata, M. J. H., Messora, M. R., Furlaneto, F. A. C., & Fucini, S. E. (2016). A new proposal for the application of platelet-rich fibrin: a case report. The International Journal of Periodontics & Restorative Dentistry, 36(3):337-345.
Publisher | Google Scholor - Mautner, K., Malanga, G. A., Smith, J., Shiple, B., Ibrahim, V., & Sampson, S. (2015). A call for a standard classification system for future biologic research: The rationale for new PRP nomenclature. PM&R, 7(4):S53-S59.
Publisher | Google Scholor - Nadjarzadeh, A., Dehghani Firouzabadi, R., Vaziri, N., Daneshbodi, H., Lotfi, M. H., & Mozafari Kermani, R. (2016). The impact of adjuvant platelet-rich plasma on clinical outcomes of intrauterine insemination cycles: a randomized clinical trial. Journal of Assisted Reproduction and Genetics, 33(1):97-103.
Publisher | Google Scholor - Mauro, A., Russo, V., Di Marcantonio, L., Berardinelli, P., Martelli, A., & Muttini, A. (2019). Platelet-Rich Plasma: A Regenerative Strategy for Cryopreserved Ovarian Tissue?. Antioxidants, 8(9):394.
Publisher | Google Scholor - Saragusty, J., & Arav, A. (2011). Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction, Fertility and Development, 24(1):14-21.
Publisher | Google Scholor - Baust, J. M., Gao, D., & Baust, J. G. (2004). Cryopreservation: An overview. CryoLetters, 25(6): 393-410.
Publisher | Google Scholor