Research Article
Unveiling The Future of Ultrasound Chemical Bath Deposition Method for Flexible Super-capacitive Electrodes: Impact of Cationic Precursors on Mno2 Flexible Electrodes
1School of Chemical Sciences, P.A.H. Solapur University, Solapur, Maharashtra, India.
2ACTREC, Kharghar, navi Mumbai, Maharashtra, India.
3Lakshmibai Bhaurao Patil Mahila Mahavidyalaya, Solapur, Maharashtra, India.
*Corresponding Author: Amarsingh Thakur, ACTREC, Kharghar, navi Mumbai, Maharashtra, India.
Citation: Deshmane A., Thakur A., Salunkhe D, Bhosale R. (2024). Unveiling The Future of Ultrasound Chemical Bath Deposition Method for Flexible Supercapacitive Electrodes: Impact of Cationic Precursors on Mno2 Flexible Electrodes. Scientific Research and Reports, BioRes Scientia Publishers. 1(3):1-18 DOI: 10.59657/2996-8550.24.012
Copyright: © 2024 Amarsingh Thakur, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: May 11, 2024 | Accepted: May 27, 2024 | Published: June 03, 2024
Abstract
MnO2 flexible electrodes (FEs) were synthesized by novel ultrasound chemical bath deposition (UCBD) using aqueous solutions of three different manganese (II) cationic precursors viz. manganese acetate, manganese chloride, and manganese nitrate. 1M aqueous NaOH solution was used as the oxidizer for each precursor. Under optimized preparative conditions, uniform MnO2 films grew on the substrates to form the FEs. XRD analysis confirmed the formation of crystalline MnO2, further corroborated by EDX. FESEM imaging revealed a transition in surface morphology from mud-like structures with manganese acetate to randomly arranged nanoflakes with manganese nitrate. Surface modification was confirmed by contact angle measurements. Cyclic voltammetry showed the specific capacitance (SC) varied with the Mn2+ cation source. Galvanostatic charge-discharge further confirmed this trend. The highest SC of 408.50 Fg-1 was exhibited by electrodes prepared with Mn (NO3)2.6H2O with 90.61 % retention in SC even after 2000 CV cycles.
Keywords: ultrasound; chemical bath; deposition method; capacitive electrodes; cationic precursors; flexible electrodes
Introduction
Redox capacitors have established themselves to contribute the major sector of charge storage devices by means of the rapid redox reactions at the electrode-electrolyte interface. Since charge storage occurs via redox transitions in the metal species rather than forming alternating layers of charge, these capacitors are identified as integral type of pseudocapacitors. Pseudocapacitors are often used for fast discharge and high-power applications [1-4]. Many electrically conducting polymers in pure [5–7] and composite/hybrid forms [8–10], along with various transition metal oxides like RuO2 [11], Co3O4 [2], Fe2O3 [4], NiO [3], Fe3O4 [12], FeOOH [13-14] Cu (OH)2 [15–17], CuO [18], etc. have been studied as electrode materials for supercapacitors. There is strong motivation to find inexpensive electrode materials that exhibit pseudocapacitance comparable to that of hydrous amorphous RuO2. MnO2 is inexpensive, abundant, and can access multiple oxidation states for redox charge storage. It exhibits a near-rectangular cyclic voltammetry curve indicative of double layer behavior and a wide potential window enabling pseudocapacitive behavior. Hence, MnO2 is a promising, affordable alternative to expensive RuO2 electrodes for supercapacitors [19-24].
Chemical bath deposition (CBD) technique is one of the various techniques for the preparation of the metal oxide/hydroxide (MO/MOH) electrodes. Literature demonstrates a plethora of work performed to synthesize the MO and MOH electrodes using CBD [25-28]. Loose bonding of the charged species on the substrate during the synthesis is one of the hurdle in obtaining the structural stability of the electrodes prepared using the CBD.
Ultrasound frequencies are often utilized for cleaning of surfaces of various objects. The vibrations at high frequency remove the foreign particles on the surface of objects. Ultrasound could also be applied during chemical growth of thin film electrodes so as to restrict the loosely bound species and provide the structural stability to the prepared electrodes. Lokhande et al. synthesized Ni2O3:Fe1.7Ni1.43O4 electrodes via ultrasound chemical bath deposition (UCBD), finding uniform film growth without loosely bound species due to ultrasound substrate vibrations [29]. Hence, we decided to synthesize flexible MnO2 electrodes via UCBD and study how manganese precursors affect surface morphology and electrochemical properties.
Materials
The chemicals used for synthesis were of analytical grade and purchased from SDFC India. Flexible stainless-steel strips (SS) (1cm × 5cm × 600μm) were used as substrate materials. Initially the thin films were deposited using pilot synthesis parameters viz. 100 ml of 0.1 M each of MnCl2•6 H2O, MnCOOH•7 H2O, and Mn(NO3)2•6 H2O as a source of Mn2+ cations and 0.1 M of NaOH as source of OH− anions.
Experimental
Electrode preparation
To prepare the substrates for thin film synthesis, the substrates were initially polished with 1200 grit emery paper. They were then treated with a 0.002 M aqueous HCl solution for 30 minutes and ultrasonicated in double distilled water for 30 minutes. This cleaning process removes contaminants and improves adhesion. These cleaned substrates were attached to an ultrasonic vibrator and immersed in a mixture containing equal volumes (100 mL each) of 0.1 M cationic and anionic precursors for 30 minutes at 200 rpm. Uniform, flexible thin film electrodes were grown on the stainless-steel substrate surfaces. The electrodes prepared using MnCl2•6H2O, MnCOOH•7H2O, and Mn(NO3)2•6H2O were labelled as MC, MA, and MN, respectively. These electrodes were analyzed for surface properties, chemical composition, and electrochemical charge storage behavior using various characterization techniques.
Characterization
X-ray diffraction analysis was carried out using the (Rigaku D/max 2550 Vb+/PC 18 kW with Cu kα λ = 0.15405 nm) diffractometer within the range 200 to 800 of diffraction angle 2θ by the step width of 0.5 deg. Surface morphological analysis was performed by using filed emission-scanning electron microscope (FE-SEM; MIRA3, TESCAN), and TEM analysis of the optimized samples was performed using TEM: CM 200, Philips respectively. Weight of the deposited materials was measured by weight difference method. Electrochemical supercapacitive behavior of the prepared electrodes was carried out using computer controlled electrochemical workstation (CHI 660 H) and with standard three electrodes cell configuration and the battery cycler (WBCS 3000). Here the prepared electrodes were (MA, MC and MN individually) were used as the working electrodes, platinum wire as a counter electrode and saturated Ag/AgCl as a reference electrode. The cyclic voltammetry (CV) was carried out within the potential window − 1.3V to 1.2V vs. Ag/AgCl in 1 M KOH at the scan rate of 100 mVs− 1 for each electrode. FE with optimum performance was subjected to the CV analyses at various scan rates from 2 mVs− 1 upto 100 mVs− 1. Galvanostatic charge-discharge (GCD) study of the prepared electrodes has been done at 1 mA current. FE with maximum SC was subjected to the GCD analysis at different applied currents. The FE with optimum electrochemical performance was also subjected to electrochemical impedance study (EIS) in the multi-frequency range 1Hz to 1MHz. The Nyquist plots were drawn from the obtained data. Electrochemical series resistance (Rs), charge transfer resistance (Rct) and Warburg impedance (Rw) were evaluated from the Nyquist plot.
Results and Discussion
Film formation mechanism
In the reaction solution bath containing the Mn salt as source of Mn2+ cations and NaOH as source of oxidizing OH- anions. As shown in figure, the Mn2+ cations combine with the OH- anions and form the Mn(OH)2. This reacts with atmospheric oxygen and gets further oxidized at room temperature to form MnO2 liberating excess water content in the form of vapours. The electrodes thus formed are hygroscopic and hence stored in the dissicator.
Structural elucidation
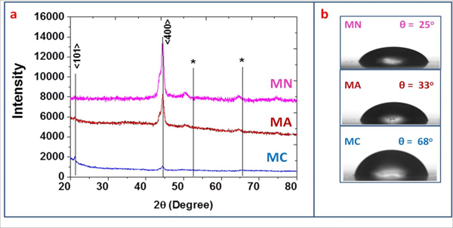
The X-Ray Diffraction (XRD) analyses of the FEs MA, MC and MN have been carried out to get crystallographic information such as orientation of the lattice planes, phase formation etc. for all the prepared samples. The electrodes exhibit peaks at 21.47o and 44.68o the observed angles and d values closely matches with the standard values mentioned in JCPDS data card 390375 indicating the formation of MnO2 lattice planes oriented along the (101) and (400) as shown in Figure. 1.a. These peaks are used to evaluate the average crystallite size using half intensity width (FWHM) of the XRD peaks using Scherer’s formula. The crystallite size along (400) plane for the samples MA, MC and MN are 14.7 nm, 14.04 nm and 14.5 nm respectively. To check whether the synthesized FEs are hydrophilic or not, the wettability study has been carried out. The contact angles for FEs MA, MC and MN are mentioned at the inset of the Figure. 1.b. It was observed that the electrode Mn exhibits lowest contact angle of 25∘.
Figure 1: a. XRD patterns; b. contact angle measurement of MC, MA and MN
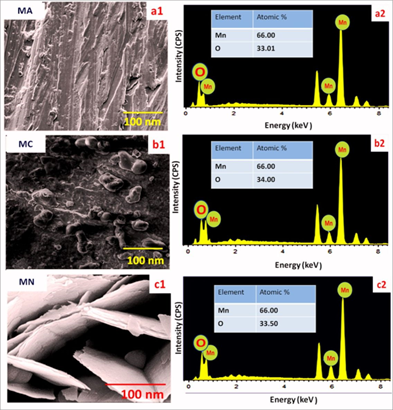
Thus, MN is more hydrophilic. This hydrophilic nature helps to improve the electrode-electrolyte interactions and hence specific capacitance (SC). Surface morphological analyses of the FEs MA, MC and MN are shown along with the elemental composition in the Figure. 2. It was observed that the FE MA exhibit dense mud-like morphology (Figure. 2.a1). The electrode MC exhibit formation of micro-globules (Figure. 2.b1) while the electrode MN exhibit the formation of nano-flakes on the surface of the electrode The average size of the flake was 110 nm (Figure. 2.c1). As the nano-flakes are randomly arranged, the surface area is higher in case of Mn electrode as compared to MA and MC electrodes.
Figure 2: a1, b1, c1 FESEM images and a2, b2, c2 EDX patterns of MA, MC and MN
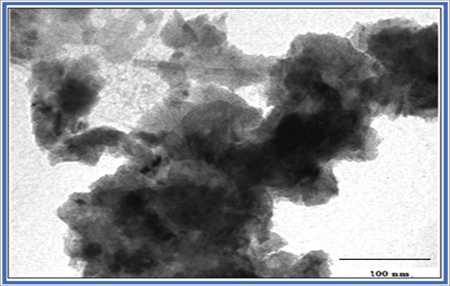
EDX patterns for MA, MC and MN are represented in Figure. 2.a.2, 2.b.2 and 2.c.2 respectively. Peaks for Mn and O observed in EDX spectra of all the three FEs prove the formation of MnO2. The atomic % analysis is mentioned in tables at the inset of respective EDX. These results are matching closely with the XRD analyses and confirm the formation of the MnO2. TEM analysis of the MN FE has been carried out (Figure. 3). As the material is very dense, we are getting darker patterns in the TEM. Some nano-rod like structures of thickness ~ 10 nm and length 110 nm have been observed.
Figure 3: TEM image of MN electrode.
Electrochemical analysis
The electrochemical analyses have been carried out in two steps.
Comparative Electrochemical analysis of the electrodes
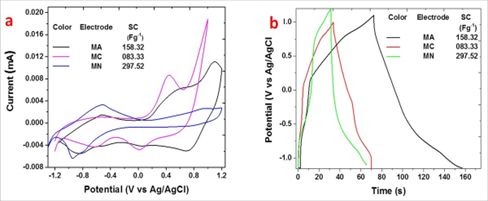
Cyclic voltammetric (CV) analyses of FEs MA, MC and MN have been carried in 20 ml aqueous solution of 1 M NaOH at potential scan rate of 100 mVs− 1 (Figure. 4.a). From CV curves, the SC values were evaluated using Eq. 1.
SC =  1
1
Figure 4: a.CV curves at 100 mVs-1; b.GCD curves at current 1 mA for MA, MC and MN in 1M NaOH
where ‘m’ be the mass of electroactive material, ‘V’ be the width of the potential window, ‘V1’ and ‘V2’ be potential limits, and be the potential scan rate. The CV curves exhibits maximum area integral (i.e., current integral) for MA, whereas the current integral for MN was minimum. Yet, as the mass of active material was smallest for MN, the value of SC was more for MN. This is in perfect accordance with the surface analyses. The observed values of SC for different FEs at a scan rate 100 mVs− 1 in 1 M NaOH are shown in table 2. The observed maximum SC is 297.52 Fg− 1 for MN. Hence Manganese Nitrate is the optimized source. Galvanostatic Charge-discharge study of electrodes MA, MC and MN has also been carried out in the same electrolyte at 1 mA applied current (Figure. 4.b).
The SC values calculated using Eq. 2 are mentioned at the inset of Figure. 2.b.
SC =  2
2
where ‘Id’ be the applied current, ‘td’ be the discharging time, ‘m’ be the mass of electroactive material, and ‘V’ is the width of the potential window. The values of SC are nearly same as those given by CV analysis. Despite of the fact that the discharge time (td) was smallest for MN electrode, it shows the maximum SC of 297.52 Figure-1. Thus the GCD corroborates that manganese nitrate is the optimized source to get the maximum SC. Thus the MN electrode was subjected to the detailed electrochemical analyses.
Detailed Electrochemical analysis of the MN electrode
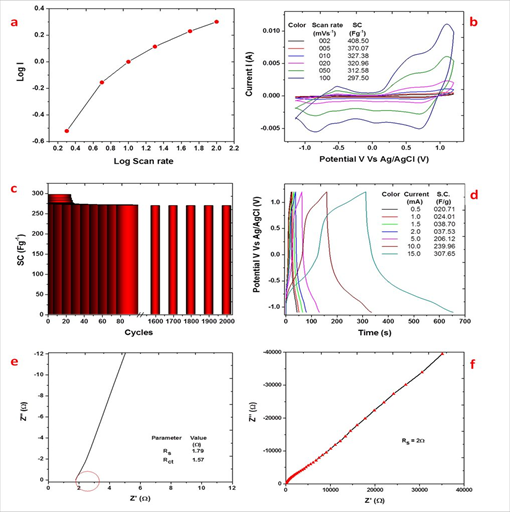
The graph of log (I) vs Log (scan rate) was plot (Figure. 5.c). The slope of the graph log (scan rate) Vs Log (I) was 0.86. This confirms that the charge storage mechanism is combination of two processes. The surface restricted adsorption-desorption of the electrolytic ions. This is accompanied with the diffusion of few ions in the bulk of the electroactive material. As the slope is tending towards the 1, the adsorption-desorption type of charge storage mechanism is dominant.
The CV analysis of MN has been carried out at different scan rates from 2 mVs-1 to 100 mVs-1 (Figure. 5.a). The values of SC at different scan rates are mentioned at the inset of Figure. 3.a. It was seen that the current integral goes on increasing with the scan rate. This increase in not in the expected proportion with the scan rate. The redox transitions are not taking place in unison with the transitions in the potential scan rate. This results in the fall in the SC with increasing scan rate. The observed maximum SC at 2 mVs-1 was 408.50 Fg-1.
To check the electrochemical cycling stability of the prepared MN electrode, it was subjected to CV at 100 mVs-1 upto 2000 cycles (Figure. 5.b). It was seen that the SC initial decreases rapidly to 92% within first 10 cycles of CV. The rate of fall in SC weakens with increasing cycles as we can see 91% retention in SC after 100 cycles. Thereafter there is no decrease in SC observed whatsoever. Thus, even after 2000 cycles, 90.61% retention in SC has been observed.
MN electrode was subjected to the GCD analysis at different currents (Figure. 5.d). The GCD curves are different from those of the ideal capacitor. The charging portion has two sub steps. Step 1 may be due to the rapid adsorption of the ions at the electrode surface. Step 2 shows rapid increase in the value of the current initially followed by the exponential part indicating the saturation of the charge at the surface as the reaction progresses. At higher applied
currents, the charge is supplied rapidly but the redox reactions take ample amount of time to take place. This might be the reason why the exponential portion appears longer at the higher currents. The discharging portion has three parts. Initial portion of small ohmic drop is seen. This might be due to the barrier resistance between the electrode and electrolyte, also the interfacial resistance between the electroactive material and the current collecting substrate. This is followed by the rapid discharging portion might be corresponding to desorption of the species from the electrode surface. Finally, the slow discharging portion this corresponds to the redox reaction between electrode and electrolyte. The SC values were calculated using formula 2 are mentioned at the inset of Figure. 3.d. It was seen that the SC value decreases with increasing currents. The observed maximum SC was 307.65 Fg-1 which is closer to that found by CV at the scan rate 100 mVs-1.
The electrochemical impedance spectroscopic study of MN electrode has been carried in the multi-frequency range 1 Hz to 1 MHz. Figure 5.e and 5.f depicts the Nyquist plots in high frequency range and whole frequency range respectively. From the Nyquist plots, the electrochemical resistance (Rs), charge transfer resistance (Rct) and Warburg impedance (Rw) were calculated. The values of Rs and Rct were as mentioned in the inset of Figure. 3.e. Low Rs of 1.79 Ω corresponds to the good conductivity of the electrolyte NaOH. Proper inter connections between the nano flakes restricts Rct to 1.57Ω. Rw was 2Ω indicating that there is less opposition to the insertion-desertion of the ions. The graph is more tilted towards the imaginary axis. This indicates the charge storage mechanism is dominated by the redox transitions resulting in the dominance of the pseudocapacitive behavior exhibited by MnO2.
Conclusion
The precursors used to make the manganese dioxide (MnO2) flexible electrodes during ultrasonic chemical bath deposition greatly influence their surface texture and thus their supercapacitive performance. The surface morphology of the fabricated flexible electrodes (FEs) changes from a mud-like texture to a surface with randomly arranged nanoflakes around 150 nm in size when manganese (II) acetate tetrahydrate [(CH3COO)Mn•6H2O] and manganese(II) nitrate hexahydrate [Mn(NO3)2•6H2O] were separately used as the cationic precursor respectively. The later increases the active surface area of the electrode, making it more hydrophilic, as evidenced by the change in contact angles. The specific capacitance ranges from 158.32 Fg− 1 for acetate-sourced MnO2 flexible electrodes to 297.52 Figue 1 for nitrate-sourced MnO2 flexible electrodes at 100 mVs− 1 during cyclic voltammetry (CV) analysis. The nitrate sourced MnO2 electrode exhibits highest SC of 408.50 Fg− 1 was exhibited by electrodes prepared with Mn(NO3)2.6H2O with 90.61% retention in SC even after 2000 CV cycles. The electrochemical series resistance (Rs) and charge transfer resistance (Rct) were found to be 1.79 Ω and 1.57 Ω, respectively. The Warburg impedance (Rw) was 2 Ω. Redox transitions of the electroactive material contribute significantly to charge storage, as the Nyquist plot shifts toward the imaginary axis. This concludes that Mn(NO3)2•6 H2O is the best precursor to fabricate MnO2 electrodes using ultrasonic chemical bath deposition.
Declarations
Conflicts of interest
Authors declare that they have no conflicts of interest regarding their roles and the work done.
Author contributions
A. A. Deshmane: Role (Methodology, Investigation)
He is the first author of the present work. He has synthesized the flexible thin film electrodes and performed the material characterizations. The author has also contributed in writing the manuscript.
A. V. Thakur: Role (Investigation, Formal analysis)
He has provided the electrochemical characterizations and have contributed to the manuscript writing.
D. J. Salunkhe:
Role (Conceptualization, Project administration)
Theme of the present work has been decided by the author and he supervised and corrected the work wherever is needed for the best results.
R. B. Bhosale: Role (Project administration)
He was the co-investigator of the work. He has done editing and corrections of the manuscript draft.
References
- B. E. Conway, Kluwer-Plenum. (1999). Electrochemical Supercapacitors Scientific Fundamentals and Technological Applications. New York, 1999.
Publisher | Google Scholor - R. C. Ambare, S. R. Bhardwaj, B. J. Lokhande. (2014). Non aqueous media dependent surface supercapacitive measurements of Co3O4 thin film electrodes prepared by spray pyrolysis, International journal of science and nature 5(4):663-668.
Publisher | Google Scholor - R. M. Kore, R.S. Mane, M. Noushad, M. R. Khan, B. J. Lokhande. (2016). Nanomorphology dependent pseudo-capacitive properties of NiO electrodes engineered through a controlled potentiodynamic electrodeposition process. R.S.C. Advances, 6:24478.
Publisher | Google Scholor - B. J. Lokhande, R.C. Ambare, R.S. Mane, S.R. Bharadwaj. (2013). Concentration-dependent electrochemical supercapacitive performance of Fe2O3. Current Applied Physics, 13:985-989.
Publisher | Google Scholor - S. P. Thokale, R. M. Kore, S. V. Kambale, A. V. Thakur, B. J. Lokhande. (2019). Aqueous route synthesis of PAni electrodes by chemical bath deposition and their cyclic voltammetric analyses at different scan rates. Macromolecular Symposia, 387(1):1800215.
Publisher | Google Scholor - V. Thakur, B. J. Lokhande. (2019). Effect of the molar concentration of pyrrole monomer on the rate of polymerization, growth and hence the electrochemical behavior of highly pristine PPy flexible electrodes, Heliyon 5(11):e02909.
Publisher | Google Scholor - V. Thakur, B. J. Lokhande. (2018). Morphological modification for optimum electrochemical performance of highly pristine polypyrrole flexible electrodes, via SILAR immersion time and fabrication of solid state supercapacitor. Portugaliae Electrochimica Acta 36(6):377-392
Publisher | Google Scholor - X. Liu, J. Yang, X. Li, et al. (2020). Fabrication of polypyrrole (PPy) nanotube electrode for supercapacitors with enhanced electrochemical performance. Journal of Materials science: Materials in Electronics, 31:581-586.
Publisher | Google Scholor - V. Thakur, B. J. Lokhande. Impact of aging on the electrochemical performance of RuO2@PPy hybrid flexible electrodes: Electrochemical study in aqueous Na2SO4. journal of electronic materials.
Publisher | Google Scholor - V. Thakur, B. J. Lokhande. (2021). Study of RuO2 incorporated PPy hybrid flexible electrodes prepared by soaking and drying technique: a novel approach Applied Physics A, 127(12):1-6.
Publisher | Google Scholor - Xia. (2012). Symmetric RuO2/RuO2 Supercapacitor Operating at 1.6 V by Using a Neutral Aqueous Electrolyte. Electrochem. Solid-State Lett, 15 A60-A63.
Publisher | Google Scholor - V. Thakur, B. J. (2017). Lokhande, Electrolytic anion affected charge storage mechanisms of Fe3O4 flexible thin film electrode in KCl and KOH: a comparative study by cyclic voltammetry and galvanostatic charge discharge. Journal of Materials Science: Materials in Electronics 216:11755-11761.
Publisher | Google Scholor - V. Thakur, B. J. (2018). Lokhande, Source molarity affected surface morphological and electrochemical transitions in binder-free FeO(OH) flexible electrodes and fabrication of symmetric supercapacitive device. Chemical Papers, 72(6):1407-1415.
Publisher | Google Scholor - V. Thakur, B. J. Lokhande. (2019) Effect of precursor bath temperature on the morphology and electrochemical performance of SILAR-synthesized PPy: FeOOH hybrid flexible electrodes. Chemical Papers, 73(4):833-841.
Publisher | Google Scholor - V. Thakur, B. J. Lokhande. (2017). Dip time-dependent SILAR synthesis and electrochemical study of highly flexible PPy-Cu (OH)2 hybrid electrodes for supercapacitors. Journal of Solid-State Electrochemistry, 21(9):2577-2584.
Publisher | Google Scholor - V. Thakur, B. J. Lokhande. (2017). Effect of dip time on the electrochemical behavior of PPy-Cu (OH)2 hybrid electrodes synthesized using pyrrole and CuSO4e-Polymers.17(2):167-173.
Publisher | Google Scholor - T. S. Ghadge, A. L. jadhav, Y. M. uplane, A. V. Thakur, S. V. Kamble, B. J. Lokhande. (2018). Controlled synthesis, structural, morphological and electrochemical study of Cu(OH)2@Cu flexible thin film electrodes prepared via aqueous-non aqueous route, Journal of materials science : materials in electronics, 32:9018-9031
Publisher | Google Scholor - S. V. Kambale, A. L. Jadhav, R. M. Kore, A. V. Thakur, B. J. Lokhande, Cyclic voltammetric study of CuO thin film electrodes prepared by automatic spray pyrolysis, Macromolecular Symposia 387,1 (2019) 1800213
Publisher | Google Scholor - Anbao Yuan, Xiuling Wang, Yuqin Wang, Jie Hu. (2010). Comparison of nano-MnO2 derived from different manganese sources, and influence of active material weight ratio on performance of nano-MnO2/activated carbon supercapacitor. Energy Conversion and Management 51:2588-2594.
Publisher | Google Scholor - J. Roberts, R. C.T. Slade. (2010). Effect of specific surface area on capacitance in asymmetric carbon/α-MnO2 supercapacitors. Electrochimica Acta, 55:7460-7469
Publisher | Google Scholor - Z. Song, W. Liu, M. Zhao, Y. Zhang, G. Liu, Chang Yu, J. Qiu. (2013). A facile template-free synthesis of a-MnO2 nanorods for supercapacitor. Journal of Alloys and Compounds, 560:151-155
Publisher | Google Scholor - Myeongjin Kim, Yongseon Hwang, Jooheon Kim. (2013). Graphene/MnO2-based composites reduced via different chemical agents for supercapacitors. Journal of Power Sources, 239:225e233
Publisher | Google Scholor - J. Yu, M. Li, X. Wang, Z. Yang. (2020). Promising High-Performance Supercapacitor Electrode Materials from MnO2 Nanosheets@Bamboo Leaf Carbon. ACS Omega, 5(26):16299-16306
Publisher | Google Scholor - Y. Kumar, S. Chopra, A. Gupta, Y. Kumar, S. J. Uke, S. P. Mardikar. (2020). Low temperature synthesis of MnO2 nanostructures for supercapacitor application. Materials Science for Energy Technologies, 3:566-574.
Publisher | Google Scholor - V. J. Mane, D. B. Malavekara, S. B. Ubale, V. C. Lokhande, C.D. (2020). Lokhande Manganese dioxide thin films deposited by chemical bath and successive ionic layer adsorption and reaction deposition methods and their supercapacitive performance. Inorganic Chemistry Communications, 115:107853
Publisher | Google Scholor - Aref, Y. W. Tang. (2014). Chemical bath deposition synthesis and electrochemical properties of MnO2 thin film: Effect of deposition time and bath temperature. Mater Sci-Pol, 32:555-564
Publisher | Google Scholor - N. R. Chodankar, G. S. Gund, D. P. Dubal, C. D. Lokhande*. (2014). Alcohol mediated growth of α-MnO2 thin films from KMnO4 precursor for high performance supercapacitors, RSC Adv., 4:61503-61513
Publisher | Google Scholor - H. Unuma, T. Kanehama, K. Yamamoto, K. Watanabe, T. Ogata. (2003). M Sugawara, Preparation of thin films of MnO2 and CeO2 by a modified chemical bath (oxidative-soak-coating) method. Journal of Materials Science, 38:255-259.
Publisher | Google Scholor - V. Thakur, N. K. Manjunath, P. B. Sarwade, B. J. Lokhande. (2023). Symmetric supercapacitor comprising Ni2O3: Fe1. 7Ni1. 43O4 electrodes prepared by ultrasound chemical bath deposition route. Materials Letters, 351:134999
Publisher | Google Scholor