Case Report
Unfolding The Diagnostic Dilemmas In MS-Like Presentations
1House Officers, Liaquat National Hospital and Medical College, Stadium Road, Karachi, Pakistan.
2Neurology Consultant, Liaquat National Hospital and Medical College, Stadium Road, Karachi, Pakistan.
3Neurology Resident, Liaquat National Hospital and Medical College, Stadium Road, Karachi, Pakistan.
*Corresponding Author: Syeda Areeba Tabassum, House Officers, Liaquat National Hospital and Medical College, Stadium Road, Karachi, Pakistan.
Citation: Ayesha A. Samad, Zaidi S, Syeda A. Tabassum, Mahza R. (2024). Unfolding The Diagnostic Dilemmas In MS-Like Presentations. Clinical Case Reports and Studies, BioRes Scientia Publishers. 6(1):1-10. DOI: 10.59657/2837-2565.brs.24.137
Copyright: © 2024 Syeda Areeba Tabassum, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: May 20, 2024 | Accepted: June 05, 2024 | Published: June 14, 2024
Abstract
This case series highlights the diagnostic challenges in differentiating neurological conditions that mimic Multiple Sclerosis (MS) in young patients. The first case involved a 23-year-old female with blurred vision and bilateral lower limb weakness initially managed for MS but later diagnosed with an infective etiology, leading to the initiation of anti-tuberculous therapy. In the second case, a young female presented with quadriplegia, initially suspected as transverse myelitis or ADEM vs MS, but the condition worsened, and repeat imaging revealed extensive medullary and cord ischemia. The third case involved a young male with paraplegia, initially diagnosed with MS, later diagnosis diverted to anti-MOG associated myelitis, leading to successful treatment and long-term management. These cases serve as important reminders of the wide spectrum of neurological conditions that can mimic MS, and they emphasize the significance of comprehensive patient history, meticulous clinical examination, and relevant laboratory investigations to avoid misdiagnosis. Seemingly straightforward clinical scenarios can be deceptively challenging. Collaboration among experienced faculty members and extensive discussions are crucial in reaching accurate diagnoses and providing appropriate treatment. As illustrated in these cases, a multidisciplinary approach involving neurology, radiology, and infectious disease specialists is essential for accurate diagnosis and management of complex neurological conditions that can present with MS-like symptoms.
Keywords: young adult; transverse myelitis; ischemia
Introduction
Multiple sclerosis (MS), presents significant difficulties in diagnosis due to its diverse and overlapping clinical manifestations. This condition involves immune-mediated damage to the myelin in the central nervous system (CNS) and commonly displays symptoms like limb weakness, sensory disturbances, visual impairments, and coordination problems. Over the past decade, there has been significant progress in the knowledge related to the differential diagnosis of multiple sclerosis (MS). Several studies have been conducted to identify disorders that are most commonly mistaken for MS in patients referred to neurologists [1-4]. Advancements in understanding CNS neuroinflammatory disorders have revealed conditions like MOG antibody-associated disease (MOGAD) and AQP4 antibody-positive neuromyelitis Optica spectrum disorder (NMOSD) that share clinical features with MS. Anti-MOG-associated myelitis is a rare disorder characterized by inflammation of the spinal cord and/or optic nerves, often affecting children and young adults. Diagnosis involves clinical evaluation, neuroimaging, and detecting anti-MOG antibodies in blood or CSF [5,6].
In the differential diagnosis of MS, CNS TB is one of the infective etiologies to consider. Differentiating between the two requires careful assessment of clinical and radiological findings. Specific radiological features of CNS TB include basilar meningeal enhancement, tuberculomas with varying representations on MRI depending on caseation, and the presence of vasculitic infarctions, especially in the Basal Ganglia. CSF studies show low sugar, high protein, and high cell count with predominant lymphocytes. PCR is a valuable and rapid tool for diagnosing tuberculous meningitis with high specificity and sensitivity reported in some studies (up to 100%) [7-12]. Paying close attention to the severity and progression of myelopathy symptoms is crucial in differentiating between inflammatory and non-inflammatory conditions. Rapid and severe deficits developing within hours may suggest spinal cord ischemia or trauma. The absence of reflexes and flaccid muscle tone can be seen in both ischemic and traumatic myelopathy. If severe acute myelitis leads to permanent loss of ambulation and neurogenic bladder requiring catheterization without recovery, multiple sclerosis (MS) is less likely. Additionally, if myelitis worsens beyond four weeks, it becomes atypical for MS, prompting consideration of other inflammatory, neoplastic, metabolic, vascular, or structural causes, necessitating further investigations for proper diagnosis and management [13-16]. "Red flags" (Table 5) in clinical and paraclinical findings prompt further investigations to rule out alternative diagnoses to MS. Clinical examination and radiographic imaging are crucial for differential diagnosis. Careful consideration reduces false-positive results, unnecessary investigations, and healthcare costs.
Case 1
A 23-year-old female experienced bilateral lower limb weakness for the past three days. Initially, she was visiting Quetta when she noticed blurring of her vision. She consulted a neurologist who administered a three-day pulse of methylprednisolone, which led to an improvement in her symptoms. Upon examination, her cognitive functions were normal. However, a focused assessment of her cranial nerves revealed reduced visual acuity and blurred disc margins. She also exhibited reduced power (3/5) in both lower limbs, with a sensory level at T12. Joint position and vibration sense were intact, but her plantar reflexes were extensor bilaterally. Initial laboratory tests and investigations returned normal results. MRI scans of her brain and cervico-dorsal spine, both with and without gadolinium enhancement, revealed multifocal abnormal T2 hyperintense signal changes in the brainstem and cerebellum. A detailed analysis of her cerebrospinal fluid (CSF) showed normal results, and CSF cultures were unremarkable. Her Visual evoked potential through flash technique was normal as she was not able to perform through checker-board. She received a pulse therapy of methylprednisolone for 5 days, which led to some improvement. She was subsequently prescribed oral steroids at a dose of 1 mg/kg and discharged.
However, a month later, she returned with worsening back pain, fever, and weakness in all four limbs, accompanied by urinary incontinence. This time, she had developed bilateral 6th nerve palsy, diminished power in all limbs (upper: 3/5, lower: 1/5), reduced reflexes, and hypotonia in all four limbs. Her plantar reflexes were bilaterally mute. A full spine MRI showed a large enhancing intramedullary lesion extending from T11 to L1, resulting in focal cord expansion. The scan also revealed extramedullary extension, edema, and meningeal enhancement, along with focal hyperintense signals in the cervical and thoracic cord at C3, C4, C5, and T1 levels. Brain MRI displayed an expansion of lesions in the brainstem and cerebellum. Considering the radiological and clinical progression of the disease, our differential diagnoses shifted more towards rapidly progressive demyelinating conditions or potential infectious processes exacerbated by steroids. We considered tuberculosis meningitis (TBM), brain abscess, CNS lymphoma, metastases, and toxoplasmosis. A repeat CSF analysis was conducted and compared with the initial sample. Her autoimmune and microbiology profiles returned negative results. She was initiated on anti-tuberculous therapy (ATT) due to worsening in her clinical condition and increase in CSF cell count. Although her cultures were negative, an assumption was made on the basis of strong radiological possibility of CNS T.B. Her symptoms significantly improved, after a month of initiation of this treatment. Therefore, we suggested to continue ATT for total of 12 months.
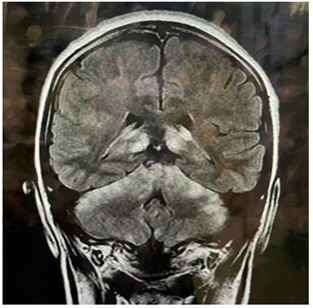
Figure 1.1: MRI brain (FLAIR) Hyperintense lesions in cerebellum and thalami.
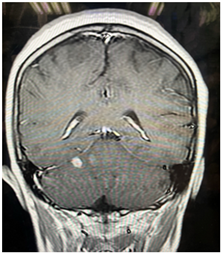
Figure 1.2: Post contrast sequence enhancing one lesion.
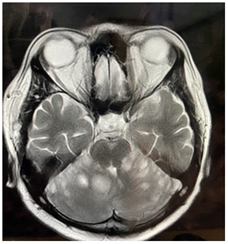
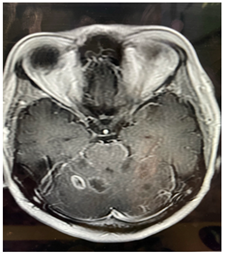
Figure 2.1: MRI Brain (T2) Hyperintense lesions in cerebellum increased in size.
Figure 2.2: lesions are giving post contrast enhancement.
Table 1: Comparison of two CSF studies.
| CSF D/R (First Sample) | CSF D/R (Repeat Sample) | Normal Values | ||
| Glucose | 69 mg/dl | Glucose | 132 mg/dl | 40-70 mg/dl |
| Protein | 31 | Protein | 104 md/dl | 20-50 mg/dl |
| WBC | Nil | WBC | 229 /cu mm | less than 5/cu mm |
| RBC | Nil | RBC | Nil | 0 |
| Neutrophils | Nil | Neutrophils | 5 % | 0 % |
| Lymphocytes | Nil | Lymphocytes | 95% | 60-70 % |
| CSF G/S | Negative | AFB Smear | Negative | |
| CSF C/S | Negative | AFB Culture | Negative | |
| OG Band | Negative | Fungus Smear | Negative | |
| Fungus Culture | Negative | |||
| MTB GeneXpert | Not Detected |
Table 2: Showing autoimmune and microbiology profile.
| Auto-Immune Profile | |||
| ANA Antibodies | Negative | ||
| Anti-smooth Muscle Antibodies | Negative | ||
| Anti-Mitochondrial Antibodies | Negative | ||
| Normal Values | |||
| Serum Anti-Cardiolipin IgG | 1.57 | IgG less than 15 GPL U/mL | |
| Serum Anti-Cardiolipin IgM | 2.67 | IgM less than 12.5 MPL U/mL | |
| Anti-MOG Antibodies | Negative | ||
| Anti-NMO Antibodies | Negative | ||
| Microbiology profile | |||
| HIV | Non-Reactive | ||
| Serum HBsAg | Non-Reactive | ||
| Serum HCV Antibody | Non-Reactive | ||
| Serum Toxoplasma IgG | Negative | ||
| Serum Toxoplasma IgM | Negative | ||
| Syphilis Screening Serology | Non-reactive | ||
| Brucella Abortus | less than 1:80 | ||
| Brucella Melitensis | less than 1:80 | ||
Case 2
A 22-year-old woman arrived at the emergency department, complaining of severe pain in her cervical region and numbness in her left arm that had been ongoing for several hours. Preceding these symptoms, she was cleaning her cupboard at a height. While in the emergency room, she suddenly experienced a fall due to the rapid onset of weakness in her lower limbs. Concurrently, she became drowsy and developed severe respiratory distress. Her heart rate dropped to 60 beats per minute, and despite receiving high-flow oxygen, her oxygen saturation plummeted to 40%, as indicated by arterial blood gas analysis. To address her severe respiratory acidosis, she had to be intubated right there in the emergency room. Upon performing a neurological examination, it was noted that she responded to pain stimuli with eye-opening, but her muscle strength was conspicuously absent (0/5) in all four limbs. Deep tendon reflexes were absent, the plantar reflexes were mute bilaterally, and horizontal nystagmus was observed. The medical team considered various potential diagnoses, including infective or demyelinating encephalomyelitis, vertebral artery dissection leading to ischemia, Bickerstaff brainstem encephalitis, or Guillain–Barré syndrome. An MRI of her cervical spine revealed subtle high signal intensity in the spinal cord, extending from the level of C2 to C7 vertebrae, suggesting the possibility of transverse myelitis. Cerebrospinal fluid (CSF) analysis indicated glucose levels of 86 mg/dl (with a serum level of 102 mg/dl), a protein concentration of 25 mg/dl, and no white blood cells or red blood cells.
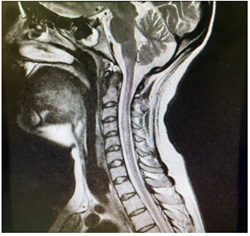
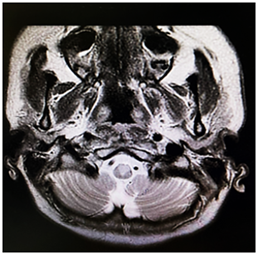
Considering the clinical and radiological presentation of transverse myelitis, the patient underwent high-dose methylprednisolone pulse therapy (1g daily for 5 days). However, after completing the steroid treatment, there was no improvement in her weakness, and she remained reliant on ventilatory support. Suspecting refractory transverse myelitis, the medical team opted for five sessions of plasma exchange, but regrettably, her condition did not ameliorate, and she remained ventilator-dependent, eventually necessitating a tracheostomy. Further investigations, including CSF oligoclonal band and NMO IgG antibody tests, yielded negative results. However, an immunoblot for antinuclear antibodies (ANA) came back positive (Ro-52+ and PM-Scl 100+), whereas tests for C-ANCA, p-ANCA, and APLA were negative. Rheumatology specialists recommended Cyclophosphamide treatment following plasma exchange. Due to the patient's unstable condition, a delay occurred in conducting repeat brain and cervical spine imaging, which took place a month later. These images revealed abnormally high signal intensity within the medulla-cervical junction, extending into the spinal cord from the level of the pons to C7 on diffusion-weighted imaging (DWI) with restricted diffusion on apparent diffusion coefficient (ADC) mapping. This indicated acute/subacute ischemia. A CT angiogram was also performed to explore the potential cause of this extensive ischemia, but the results came back normal. Vertebral artery dissection due to neck manipulation was suspected as the likely underlying cause of anterior spinal artery infarction. The patient was ultimately discharged home with ventilator support.
Figure 3.1: MRI cervical spine showing hyperintense lesion extending from medulla to C7.
Figure 3.2: MRI brain sequence showing hyperintense area in the medial medulla.
Case 3
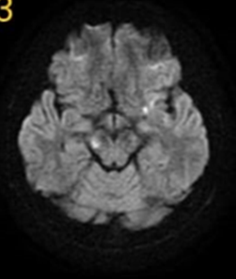
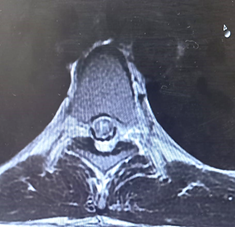
A 25-year-old man came to our clinic with a history of bilateral lower limb weakness that had been ongoing for a year. It all began with a sudden onset of mild numbness in his left lower limb, which resolved within a month with symptomatic treatment. However, after a year, he experienced numbness in both lower limbs, followed by weakness and urinary incontinence. He was initially treated with a high dose of methylprednisolone, which helped improve his symptoms over three weeks, allowing him to resume his daily activities. Unfortunately, his symptoms returned two months later, with weakness in both lower limbs and bladder dysfunction. Even with early steroid treatment, his condition did not improve. Brain MRI showed a small area of abnormal signal in the cortex, while MRI scans of the cervical and thoracic spine appeared normal. A cerebrospinal fluid (CSF) analysis showed pleocytosis with predominantly lymphocytes, leading to the initiation of anti-tuberculous treatment. However, there was no improvement, and he discontinued this treatment. Upon examination in our clinic, his higher mental functions were intact. Cranial nerve examination revealed right relative afferent pupillary reflex (RAPD) and mild horizontal nystagmus. Motor assessment showed normal muscle bulk, increased tone in the lower extremities, and weakness in the left lower limb (1/5) and right lower limb (3/5), while the upper limbs were normal (5/5). Reflexes were brisk throughout the body, with positive clonus bilaterally, and the plantar reflexes were extensor. Sensory loss was at the level of D10. Given the multifocal nature of his symptoms, affecting the optic nerves, cerebellum, and thoracic spine, a comprehensive workup was performed, including laboratory tests, echocardiogram, and hypercoagulable screening, all of which yielded normal results. A repeat MRI of the brain showed hyperintense lesions in the midbrain, along with unspecified bright spotty lesions in cortical and subcortical areas. Magnetic resonance angiography (MRA) was normal, ruling out vasculitis. An MRI of the thoracic spine on T2-weighted imaging showed a high signal in the central grey matter from D3 to D6, with patchy post-contrast enhancement at D6. The initial treatment approach involved a five-day course of high-dose methylprednisolone followed by five sessions of plasmapheresis. Unfortunately, we were unable to test for anti-neuromyelitis Optica (NMO) IgG and anti-myelin oligodendrocyte glycoprotein (MOG) antibodies in our city. Oligoclonal bands in the serum and CSF were matching. Given these findings, we provisionally diagnosed the patient with anti-MOG-associated myelitis. With treatment, his symptoms significantly improved, and he was discharged home.
Figure 4: MRI brain DWI sequence showing nonspecific areas of T2 shine through.
Figure 5: MRI thoracic spine transverse section showing hyperintense area specifically involving the central grey matter.
Table 3: Comparison of CSF studies.
| CSF D/R (First Sample) | CSF D/R (Repeat Sample) | Normal Values | ||
| Glucose | 57 mg/dl | Glucose | 59 mg/dl | 40-70 mg/dl |
| Protein | less than 0.2 gm/dl | Protein | 42 mg/dl | 20-50 mg/dl |
| WBC | 43 /cu mm | WBC | 107 /cu mm | less than 5/cu mm |
| RBC | Nil | RBC | 4200 /cu mm | 0 |
| Neutrophils | 7 % | Neutrophils | 10 % | 0 % |
| Lymphocytes | 93% | Lymphocytes | - | 60-70 % |
| OG Bands | Negative | CSF G/S | Negative | |
| CSF C/S | Negative | |||
| MTB-PCR | Not Detected | |||
| HSV-PCR | Not Detected | |||
| AFB Culture | Not Detected |
Table 4: Laboratory & Hypercoagulable Workup.
| Normal values | |||
| TLC | 9.07 | 4.0 - 11.0 x  /l /l | |
| Creatinine | 0.57 | 0.7 – 1.3 mg/Dl | |
| Potassium | 3.30 | 3.5 – 5.2 mEq/L | |
| Serum Albumin | 3.66 | 3.4 – 5.4 g/Dl | |
| Serum Bilirubin | 0.88 | 0.1 – 1.2 mg/dL | |
| SGPT | 16 | 7 – 56 U/L | |
| ALP | 62 | 44 – 147 IU/L | |
| GGT | 43 | 5 – 40 U/L | |
| AST | 36 | 8 – 33 U/L | |
| CRP | 8.57 | 3 – 1.0 mg/dL | |
| ANA Profile | Negative | ||
| Protein C | less than 150 (70-150%) | ||
| Protein S | 140 (60-150%) | ||
| Anti-Thrombin | 125 (80-130%) | ||
| Factor V | 0.95 | ||
Discussion
The progression and severity of neurological symptoms, in the first presented case, were not in line with the usual course of any demyelination pathology like multiple sclerosis. While MS relapses can lead to transient neurological deficits, the extensive progression and worsening of symptoms, especially with steroids administration, were indicative of a more aggressive and potentially infective process. The importance of repeat imaging and comprehensive CSF analysis cannot be understated. There were several clinical and radiological features which helped us in reaching the seemingly common but critical possibility of CNS Tuberculosis. Certain radiological features in our case includes complete ring enhancement, basilar meningeal enhancement and diffusion restriction in certain areas of the brain like corpus callosum. CSF studies showed high cell count with predominant lymphocytes also favored the infective process. MTB PCR was negative in our case. AFB culture was not sent in the repeated sample. Similarly spinal involvement of cord with extramedullary extension and meningeal enhancement was also alarming towards an alternate diagnosis. Though, anti-tuberculous therapy response was judged for few weeks only as she left for her hometown in Sydney but found to have improvement subjectively.
The second case highlights the complexity of diagnosing demyelinating disorders that can mimic ischemic events, such as acute/subacute ischemia in the medulla-cervical junction. The initial clinical presentation with cervical pain and numbness followed by severe limb weakness and respiratory distress suggested a potential lesion localization at the high cervical region above C3-C4. We assumed the possible mechanism to be myelitis. However, the failure to respond to steroids, plasma exchange, and immunomodulatory therapies raised concerns about the accuracy of the initial diagnosis. Subsequent imaging revealed abnormalities consistent with spinal cord ischemia prompting reconsideration of the differential diagnosis. If the initial MRI is negative but clinical suspicion is high, repeat imaging is recommended, because 60% were positive on repeat imaging. Spinal cord ischemia is rare accounting for 1% of all strokes. Hyperextension or neck manipulation is the leading cause of vertebral artery dissection. Upon exploring the possible causative mechanism his father revealed that she was cleaning the upper portion of her cupboard which may triggered the event. Vertebral dissection can be seen in 4% to 10% of Spinal cord ischemia, but this number is higher in younger populations. 16% of dissections do not show up on CT angiogram if delayed for a month as can be seen in our case [17].
In the third case, the patient's clinical presentation, along with distinct radiological patterns observed in the spine, strongly indicated a potential diagnosis of MOG myelitis. This condition, though resembling infective etiologies due to an elevated cell count in the cerebrospinal fluid (CSF), is recognized to exhibit higher CSF cell counts, sometimes exceeding 100 cells per cubic millimeter. Furthermore, the reviewed literature consistently highlighted that MOG myelitis often manifests with characteristic radiological findings, including "bright spotty lesions" on T2-weighted sequences in brain MRI scans. While brain MRI scans may appear unremarkable, spinal MRI scans typically display lesions selectively affecting the deep grey matter of the spinal cord [18]. The critical role of a comprehensive understanding of the clinical context in conjunction with radiological correlations, which ultimately guided the medical team toward a definitive diagnosis. Despite the inherent challenge posed by the absence of MOG antibody testing in our healthcare setting, the successful management of the patient's condition underscores the significance of relying on clinical judgment and radiological evidence in regions with limited diagnostic resources for rare neurological disorders. In all three cases, the role of detailed clinical assessment, radiological correlation, and collaboration among experienced faculty proved crucial in reaching accurate diagnoses and providing appropriate treatment. It is essential to stay updated with the latest medical literature and diagnostic tools to enhance diagnostic accuracy and improve patient outcomes.
Table 5: RED-FLAGS of multiple sclerosis [19].
| Radiological | CSF Study | Para-Clinical |
| Meningeal Enhancement | Pleocytosis of More Than 50 cell/cu mm | Bilateral Optic Neuritis |
| Increasing Lesions | Significantly Elevated CSF Protein > 100 mg/dl | Complete Gaze Palsy |
| Indistinct Lesions | Presence of Atypical Cells (Neutrophils/Eosinophils) | Intractable Nausea, Vomiting, Hiccups |
| Micro Bleeds | Absence of CSF Oligoclonal Bands | Complete Transverse Myelopathy with Bilateral Motor and Sensory Symptoms |
| Infarcts | Encephalopathy, Cognitive Decline, Headache, Meningism, Constitutional Symptoms | |
| Complete Ring-Enhancement | ||
| Symmetric Lesions | ||
| Sparing of U-Fibers | ||
| Siderosis | ||
| Longitudinally Extensive Lesions in the Spinal Cord |
Declarations
Conflict of Interest: None.
Consent was Taken: Yes.
Financial Support: None.
References
- Solomon AJ, Bourdette DN, Cross AH, et al. (2016). The contemporary spectrum of multiple sclerosis misdiagnosis: a multicenter study. Neurology. 87:1393-1399.
Publisher | Google Scholor - Kaisey M, Solomon AJ, Luu M, Giesser BS, Sicotte NL. (2019). Incidence of multiple sclerosis misdiagnosis in referrals to two academic centres. Mult Scler Relat Disord. 30:51-56.
Publisher | Google Scholor - Midaglia L, Sastre-Garriga J, Pappolla A, et al. (2021). The frequency and characteristics of MS misdiagnosis in patients referred to the multiple sclerosis center of Catalonia. Mult Scler. 27:913-921.
Publisher | Google Scholor - Gaitán MI, Sanchez M, Farez MF, et al. (2022). The Frequency and Characteristics of Multiple Sclerosis Misdiagnosis in Latin America: A Referral Centre Study in Buenos Aires, Argentina. Mult Scler. 28:1373-1381.
Publisher | Google Scholor - Reindl M. (2015). MOG Antibody-Associated Diseases. Neurol Neuroimmunol Neuroinflamm. 2(1):e803.
Publisher | Google Scholor - Baumann M, Sahin K, Lechner C, Hennes EM, Schanda K, et al. (2015). Clinical and neuroradiological differences of paediatric acute disseminating encephalomyelitis with and without antibodies to the myelin oligodendrocyte glycoprotein. J Neurol Neurosurg Psychiatry. 86(3):265-272.
Publisher | Google Scholor - Wilkinson RJ, Rohlwink U, Misra UK, Van Crevel R, Mai NT, et al. (2017). Tuberculous meningitis. Nature Reviews Neurology. 13(10):581-598.
Publisher | Google Scholor - Johansen IS, Lundgren B, Tabak F, Petrini B, Hosoglu S, et al. (2004). Improved sensitivity of nucleic acid amplification for rapid diagnosis of tuberculous meningitis. Journal of Clinical Microbiology. 42(7):3036-3040.
Publisher | Google Scholor - World Health Organization. (2013). World Health Organization Global Tuberculosis Report 2013. World Health Organization: Geneva, Switzerland.
Publisher | Google Scholor - Pai M, Flores LL, Pai N, Hubbard A, Riley LW, et al. (2003). Diagnostic accuracy of nucleic acid amplification tests for tuberculous meningitis: a systematic review and meta-analysis. The Lancet Infectious Diseases. 3(10):633-643.
Publisher | Google Scholor - Phypers M, Harris T, Power C. (2006). CNS tuberculosis: a longitudinal analysis of epidemiological and clinical features. The International Journal of Tuberculosis and Lung Disease. 10(1):99-103.
Publisher | Google Scholor - Donald PR, Schoeman JF. (2004). Tuberculous Meningitis. Tehe New England Journal of Medicine. 351(17):1719-1720.
Publisher | Google Scholor - Lopez Chiriboga S, Flanagan EP. (2021). Myelitis and other autoimmune myelopathies. Continuum. 27:62-92.
Publisher | Google Scholor - Transverse Myelitis Consortium Working Group. (2002). Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 59:499-505.
Publisher | Google Scholor - Barreras P, Fitzgerald KC, Mealy MA, et al. (2018). Clinical biomarkers differentiate myelitis from vascular and other causes of myelopathy. Neurology. 90:e12-e21.
Publisher | Google Scholor - Zalewski NL, Rabinstein AA, Krecke KN, et al. Characteristics of spontaneous spinal cord infarction and proposed diagnostic criteria. JAMA Neurol 2019; 76: 56–63.
Publisher | Google Scholor - Walzl D, Solomon AJ, Stone J. Montalvo M, Bayer A, et al. (2018). Spinal Cord Infarction Because of Spontaneous Vertebral Artery Dissection. Stroke. 49(11):e314-e317.
Publisher | Google Scholor - Riskind, Peter N. (2009). Multiple Sclerosis’ Continuum Lifelong Learning. 15(2):148-178.
Publisher | Google Scholor - Geraldes R, Ciccarelli O, Barkhof F, De Stefano N, Enzinger C, et al. (2018). The current role of MRI in differentiating multiple sclerosis from its imaging mimics. Nat Rev Neurol. 14(4):199-213.
Publisher | Google Scholor