Research Article
Trends in Pancreatic Cancer Related Mortality in the United States from 1999-2020
1Army Medical College, Pakistan.
2Rehman Medical College, Pakistan.
*Corresponding Author: Shandana Younas, Rehman Medical College, Pakistan.
Citation: Muhammad A. I. Chughtai, Younas S. (2023). Trends in Pancreatic Cancer Related Mortality in the United States from 1999-2020. Clinical Case Reports and Studies, BioRes Scientia Publishers. 3(5):1-9. DOI: 10.59657/2837-2565.brs.23.080
Copyright: © 2023 Shandana Younas, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: October 07, 2023 | Accepted: October 25, 2023 | Published: November 06, 2023
Abstract
Background: Pancreatic cancer related mortality trends in the U.S. population have not been explored recently and the availability of the CDC WONDER database provides us the opportunity to do so.
Objectives: The purpose of this study was to determine the directionality of trends and the regional differences in pancreatic cancer related mortality in the United States from 1999 to 2020.
Methods: Death certificates from the CDC WONDER (Centers for Disease Control and Prevention Wide-Ranging Online Data for Epidemiological Research) database were inspected from 1999 to 2020 for pancreatic cancer related mortality in all age groups of the US population. Age adjusted mortality rates (AAMRs) per 100,000 persons and annual percentage change (APC) were calculated and compared with respect to year, sex, race/ethnicity and geographic region.
Results: Between 1999 and 2020, 847589 Pancreatic cancer-related deaths occurred among all age groups of the U.S. population. The overall population AAMR increased from 11.2 in 1999 to 11.7 in 2020 (APC: 0.26; 95% CI: 0.20-0.32). Men had persistently higher AAMRs than women from 1999 (AAMR men: 12.9 vs women: 9.7) to 2020 (AAMR men: 13.6 vs women: 10.2). Non-Hispanic (NH) Black or African American population had the highest overall AAMR (14.5), followed by NH White (11.4), Hispanic or Latino (8.9), NH American Indian or Alaska Native (8.5), and NH Asian or Pacific Islander adults (8.0). AAMRs also varied by region (overall AAMR: Northeast: 11.9; Midwest 11.7; South: 11.3; West: 10.8). In comparing States, District of Columbia had the highest overall AAMR (14.0) and Utah the lowest (9.8). Comparison of 5-year age groups revealed the highest crude death rate in the 80-84 age group (93.5 per 100,000 persons), with an appreciable rise through the middle and older ages. We then analyzed data for adults between the ages of 45 and 84 and saw similar trends as before.
Conclusion: Pancreatic Cancer- related mortality in U.S. has increased since 1999. Despite an overall decreasing trend of AAMRs from 1999 to 2020 for the NH Black or African American population, this category still had the highest AAMRs of the races/ethnicities. Men had persistently higher AAMRs than women. A disproportionate AAMR distribution pertaining to the States was exhibited. These differences warrant further investigation and focused approaches are needed to counter these mortality trends.
Keywords: cancer; pancreas; AAMR
Introduction
Across the globe, the prevalence of pancreatic cancer is on the rise and has surged by over two-fold in the last three decades [1]. In 2018, pancreatic cancer ranked as the seventh leading contributor to cancer-related deaths worldwide [2]. However, Pancreatic ductal adenocarcinoma (PDAC) ranks as the third main contributor to cancer-related mortality in the United States, and it is associated with a mere 10% survival rate over a five-year period [3]. Pancreatic adenocarcinoma is responsible for approximately 90% of all Pancreatic Cancers [4]. The estimated new cases of Pancreatic cancer in 2023 as of Sep23’ are 64,050 which is 3.3% of all new cancer cases in this period and the estimated deaths from from pancreatic cancer in 2023 as of Sep23’ are 50,550 which is 8.3% of all cancer deaths in this period [5]. In a cross-sectional cancer projection study conducted by Rahib et al. in 2021, it was shown that by the year 2040, lung, prostate, liver, and pancreas were expected to be the leading sources of cancer-related fatalities among men. Likewise, for women, the principal contributors to cancer-related mortality in 2040 were projected to be lung, breast, pancreas, and uterine cancer [6]. Awareness of the demographic and regional patterns in relation to Pancreatic Cancer mortality can aid in the identification of populations at highest risk so that timely interventions can be offered. Therefore, we sought to evaluate these differences in Pancreatic Malignancy associated AAMRs from 1999 to 2020.
Methods
Study Setting and Population
In this study, death certificate data were retrieved from the CDC WONDER (Centers for Disease Control and Prevention Wide-Ranging OnLine Data for Epidemiologic Research) database (7) and investigated from 1999 to 2020 for Pancreatic cancer- related mortality in the overall population and separately in adults aged 45 -84 years using codes from the International Statistical Classification of Diseases and Related Health Problems-10th Revi- sion (ICD-10) as follows: C25.0 (Head of pancreas - Malignant neoplasms); C25.1 (Body of pancreas - Malignant neoplasms); C25.2 (Tail of pancreas - Malignant neoplasms); C25.3 (Pancreatic duct - Malignant neoplasms); C25.4 (Endocrine pancreas - Malignant neoplasms); C25.7 (Other parts of pancreas - Malignant neoplasms); C25.8 (Overlapping lesion of pancreas - Malignant neoplasms); C25.9 (Pancreas, unspecified - Malignant neoplasms). The Multiple Cause-of-Death Public Use record death certificates were examined to select Pancreatic Neoplasia-related deaths, identified as those with Pancreatic Cancer reported anywhere on the death certification either as a contributing or underlying cause of death. A sensitivity analysis was also conducted following the STROBE (Strengthening the Reporting of Obser- vational Studies in Epidemiology) guidelines for reporting.
Data Abstraction
Data for population, year, geographical region and states were abstracted. Demographics including sex, age, and race/ethnicity were isolated. Race/ethnicity was classified as Hispanic or Latino, non-Hispanic (NH) White, NH Black or African American, NH American Indian or Alaskan Native and NH Asian or Pacific Islander. This information has been extracted from reported data on death certificates and has previously been used in the analyses of mortality trends pertaining to several causal factors of death from the CDC WONDER database [8,9]. Regions were classified into Northeast, Midwest, South, and West according to the U.S. Census Bureau definitions [10].
Statistical Analysis
To examine national trends in Pancreatic Cancer related mortality, we calculated crude and age- adjusted mortality rates (AAMRs) per 100,000 population from 1999 to 2020 by year, sex, race/ethnicity and state with 95% CIs. Crude mortality rates were analyzed when comparing the AAMRs with respect to different age groups and were determined by dividing the number of Pancreatic Cancer related deaths by the corresponding U.S. population of that year. AAMRs were calculated by standardizing Pancreatic Cancer related deaths to the year 2000 U.S. population. To quantify annual AAMR trends, the Joinpoint Regression Program (Version 5.0.2; National Cancer Institute) was used to determine the annual percent change (APC) with 95% CI in AAMR [11]. This approach detects noteworthy fluctuations in AAMR (Age-Adjusted Mortality Rate) over a period by employing log-linear regression models in cases where there is temporal variation. APCs (Annual Percent Changes) were categorized as rising or falling if the slope representing the mortality change was significantly distinct from zero through a two-tailed t-test. A significance level of P less than 0.05 was considered statistically significant.
Results
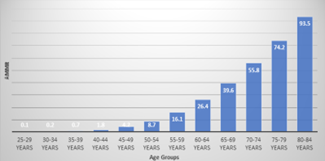
A total of 847,589 Pancreatic Cancer related deaths occurred among the overall U.S. National population between 1999 and 2020. Of these, 703,360 deaths occurred in the adult population aged 45-84 years old. Crude death rates could not be calculated for 85+ years old population because total population data for this age group was not available [12] and the extracted death rates are flagged as unreliable for ages 1-14 as the rate is calculated with a numerator of 20 or less [13). In addition, distinctly higher death tolls could be appreciated with the population entering middle and older ages age as shown in figure 1. Therefore, we shall be analyzing the overall population data recruited from the CDC WONDER database and simultaneously doing a sensitivity analysis of the age category 45-84 years.
Figure 1: Average AAMRs for 5-yr Age Groups 1999-2000
Annual Trends for Pancreatic Cancer-Related Aamr
The overall population AAMR increased from 11.2 in 1999 to 11.7 in 2020 (APC: 0.26; 95% CI: 0.20-0.32). AAMR for adults aged 45-84 increased from 27.8 in 1999 to 28.4 in 2014 (APC: 0.24; 95% CI: -0.41-0.35) and increased further to 29.6 in 2020 (APC: 0.58; 95% CI: 0.35-1.25). The Average APC (AAPC) for this subpopulation on sensitivity analysis was 0.33 (95% CI: 0.26-0.41) as shown in Figure 2.
Figure 2: AAMRs Ages 45-84; 1999-2020
Pancreatic Cancer-Related Aamr Stratified by Geographic Region
A difference in AAMR was observed in different states for the overall population and subgroup 45-89 age category, with the AAMRs ranging from 14.0 (95% CI: 13.4-14.7) in District of Columbia to 9.8 (95% CI: 9.5-10) in Utah for all ages and 35.3 (95% CI: 33.5-37.1) in District of Columbia to 24.3 (95% CI: 23.5-25.1) in Utah for ages 45-84 as shown in Figure 3.
Figure 3: State-wise Distribution of Pancreatic Cancer Related AAMR in the U.S. population aged 45-89 from 1999-2020.
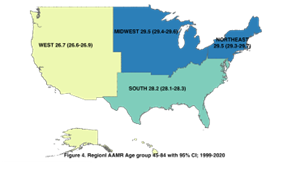
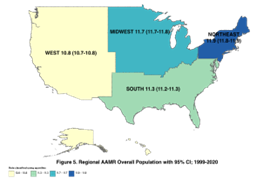
The mortality trends with regards to the four geographical regions of the U.S. are as demonstrated in figure 4 & 5 for the age group 45-84 and overall population (comprising all age groups), respectively.
Figure 4
Figure 5
Pancreatic cancer-related AAMR stratified by sex
In the overall population, males had higher AAMRs than females throughout the study period (overall AAMR men: 13.2; 95% CI: 13.1-13.2; women: 9.9; 95% CI: 9.9-10). In 1999, the AAMR for all population males was 12.9 (95% CI: 12.7-13.2), which increased to 13.6 in 2020 (APC: 0.28; 95% CI: 0.21-0.35). Similarly, the AAMR for females in 1999 was 9.7 (95% CI: 9.6-9.9), which significantly rose to 10.2 in 2020 (APC: 0.23; 95% CI: 0.15-0.30).
In the 45-84 age group population, males had higher AAMRs than females throughout the study period (overall AAMR men: 33.1; 95% CI: 33.0-33.2; women 22.4; 95% CI: 22.3-22.5). In 1999, the AAMR for all population males was 32.6 (95% CI: 32.0-33.2), which increased to 32.6 in 2013 (APC: 0.16; 95% CI: -0.42-0.29) and further increased to 34.5 in 2020(APC: 0.66; 95% CI: 0.40-1.5). Similarly, the AAMR for females after the age categorization was 24 (95% CI: 23.5-24.4) in 1999, which significantly increased to 25.3 in 2020 (APC: 0.26; 95% CI: 0.19-0.33). These data have been demonstrated graphically in Figure 6.
Figure 6: AAMRs for Overall Population vs Age Group 45-84: Stratified by sex
Pancreatic cancer-related aamr stratified by race/ethnicity
Figure 7: AAMRs for Population aged 45-84; Stratified by Race/Ethnicity
Figure 8: AAMRs for Overall Population (all ages); Stratified by Race/Ethnicity
Trends in pancreatic cancer related mortality for population age group 45-84
AAMRs were highest among NH Black or African American patients followed by NH White, Hispanic or Latino, NH American Indian or Alaska Native, and NH Asian or Pacific Islander populations (overall AAMR NH Black or African American: 14.5; 95% CI: 14.4-14.6; NH White: 11.42; 95% CI: 11.39-11.45; Hispanic or Latino: 8.94; 95% CI: 8.85-9.02; NH American Indian or Alaska Native: 8.5; 95% CI: 8.2-8.8; NH Asian or Pacific Islander: 7.96; 95% CI: 7.85-8.01). In terms of patterns, the AAMR for NH Black or African American dropped from 1999 to 2002 (APC: -1.8; CI -3.8 to -0.2) and there were no statistically significant changes from then up till 2020. Regardless, the Average APC (AAPC) was -0.2 (Cl: -0.40 to -0.04), confirming a downward trend of AAMR from 15.2 in 1999 to 14.3 in 2020. The AAMR for Hispanic or Latino population did not reveal statistically significant changes until 2017 after which there was a significant rise till 2020 (APC: 2.4; CI 0.4-5.0). There was an overall rise in AAMR from 8.4 in 1999 to 9.4 in 2020 (AAPC: 0.5; CI 0.2-0.8). Amongst the AAMRs for NH White, NH American Indian or Alaska Native and NH Asian or Pacific Islander population, only NH White population showed a significant rise from 11.0 in 1999 to 11.9 in 2020 (APC: 0.5; Cl 0.40-0.53).
Trends in pancreatic cancer related mortality for overall population (all age groups)
Similar trends in similar general trajectories were appreciated as displayed in Figures 7 and 8.
Discussion
In our examination of mortality data spanning two decades, we present a few noteworthy findings. First and foremost, it's crucial to highlight that there has been no overall decline in Pancreatic cancer related mortality rates from 1999-2020. Pancreatic cancer has consistently remained one of the prominent diseases leading to mortality in the United States, and over a span of 22 years, we have observed a rise in AAMRs for both men and women. One possible explanation for these statistics could be that majority of patients do not display noticeable symptoms through disease progression to advanced metastatic cancer, where tumor cells exhibit significant invasiveness. Detecting the cancer at an early stage poses a considerable challenge [14]. In addition, majority of patients ultimately experience a recurrence, even following an aggressive treatment approach, resulting in only 12.5% survival rate over a five-year period (2013-2019) [5].
Mortality rate data for both sexes exhibited an upward trend and men had consistently higher AAMRs than women over the course of 22 years. The difference in mortality rates might be attempted to be clarified by the global variation in the incidence of Pancreatic cancer between the sexes, incidence in men being higher than women [15,16]. One potential explanation could be that women tend to have higher levels of certain hormones, which could have a protective effect against pancreatic cancer [17). This was supported by Sadr-Azodi et al.'s paired cohort study, which found that women who received menopausal hormone therapy had a 23% lower prevalence of pancreatic cancer compared to those who did not receive MHT. Additionally, MHT for 1-2 years reduced the prevalence by 35%, while MHT for over 3 years led to a 60% reduction in prevalence [18).
Previous, more dated research data supports our conclusions about the Black or African American population having the highest AAMRs among the five racial groups [19,20]. At present, there is a shortage of studies addressing this racial disparity, making it a critical research gap area that needs our focus to curtail the number of deaths from pancreatic cancer. Efforts to comprehend the causes behind this inequality have proposed that when it comes to men, the well-established risk factors, primarily cigarette smoking and diabetes mellitus, account for nearly all of the difference in pancreatic cancer rates between black and white individuals. On the other hand, among women, it seems that additional factors play a role, particularly substantial alcohol consumption and high body mass index [21]. The incidence of pancreatic cancer among Black individuals residing in Africa seems to be notably lower when contrasted with the rates observed among African-Americans [22]. Notably, among all the population subsets examined, the only one to show a decline in mortality from Pancreatic cancer was the Black or African American community. To our understanding, there has been relatively little investigation into this specific aspect of Pancreatic cancer, which provides additional rationale for conducting a more thorough analysis of the statistics related to pancreatic cancer within the Black or African American population in the United States.
The geographic variation in AAMRs, with District of Columbia having the highest and Utah having the lowest AAMRs; albeit both displaying a rising trend over the years, bears resemblance to a study by Hao Y et al. discussing the geographic patterns of cancer related mortality in the U.S. by congressional districts, in which, the following was brought to light: In the case of men, death rates (per 100,000 person-years) range from 186.3 in Utah's congressional district #3 to 343.7 in the District of Columbia. Similarly, for women, the rates span from 123.4 in Utah's congressional district #1 to 217.4 in Pennsylvania's congressional district #2 [23]. This data underscores how death rate patterns vary by area and warrants further county wise analysis to delve deeper into resolving any district specific barriers to the provision of healthcare services.
study Limitations
Firstly, due to reliance on ICD codes and death certificates, there is a chance for inaccurate reporting or the omission of cases. Secondly, the database lacks details on clinical factors that could be helpful in the characterization of pancreatic cancer. Thirdly, information regarding pancreatic cancer management is absent. Lastly, data related to socioeconomic factors influencing health, which can impact access to healthcare, is also unavailable.
References
- Pourshams A, Sepanlou SG, Ikuta KS, Bisignano C, Safiri S, Roshandel G, et al. (2017). The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet Gastroenterology & Hepatology.
Publisher | Google Scholor - Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin., 68(6):394–424.
Publisher | Google Scholor - Siegel RL, Miller KD, Fuchs HE, Jemal A. (2021). Cancer Statistics, 2021. CA Cancer J Clin., 71(1):7-33.
Publisher | Google Scholor - Pishvaian MJ, Brody JR. (2017). Therapeutic Implications of Molecular Subtyping for Pancreatic Cancer. Oncology (Williston Park), 15;31(3):159-66,168.
Publisher | Google Scholor - (2023). National Cancer Institute. 2023
Publisher | Google Scholor - Rahib L, Wehner MR, Matrisian LM, Nead KT. (2021). Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw Open, 1;4(4):e214708.
Publisher | Google Scholor - Centers for Disease Control and Prevention. (2023) National Center for Health Statistics. Multiple cause of death 1999-2019 on CDC (2023). ONDER on- line Database, released in 2020. Data are from the Multiple Cause of Death Files, 1999-2019, as compiled from data provided by the 57 vital sta-tistics jurisdictions through the Vital Statistics Cooperative Program.
Publisher | Google Scholor - Maqsood MH, Talha KM, Minhas AMK, Fudim M, Khan SS, et al. (2023). CDC-WONDER Database Analysis of COVID-19 and cardiovascular disease-Related Mortality. J Am Coll Cardiol., 81(17):1743-1745.
Publisher | Google Scholor - Patel R, Arisoyin AE, Okoronkwo OU, Aruoture S, Okobi OE, et al. (2023). Trends and Factors Associated with the Mortality Rate of Depressive Episodes: An Analysis of the CDC Wide-Ranging Online Data for Epidemiological Research (WONDER) Database. Cureus., 15(7):e41627.
Publisher | Google Scholor - Minhas AMK, Gupta K, Jain V, Kakar TS, Merchant AT, Shapiro MD, (2023). Trends in Cardiovascular Mortality in the United States from 1968 to 2019: Analysis of the CDC Wonder Database. Eur J Prev Cardiol.
Publisher | Google Scholor - Ingram DD, Franco SJ. (2013). NCHS Urban-Rural Classification Scheme for Counties. Vital Health Stat, (166):1-73.
Publisher | Google Scholor - Joinpoint trend analysis software. (2023). Joinpoint regression program, Surveillance Research Program. National Cancer Institute.
Publisher | Google Scholor - CDC. (2020). Multiple Cause of Death 1999 – 2020
Publisher | Google Scholor - Gillen S, Schuster T, Meyer Zum Buschenfelde C, Friess H, Kleeff J. (2010). Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Medicine, 7(4):e1000267
Publisher | Google Scholor - McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. (2018). Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol, 24(43):4846-4861.
Publisher | Google Scholor - Cronin KA, Scott S, Firth AU, Sung H, Henley SJ, (2022). Annual report to the nation on the status of cancer, part 1: National cancer statistics. Cancer, 128(24):4251-4284.
Publisher | Google Scholor - Masoudi S, Momayez Sanat Z, Mahmud Saleh A, Nozari N, Ghamarzad N. Menstrual and reproductive factors and risk of pancreatic cancer in women. Middle East J Dig Dis. 9(3):146-149.
Publisher | Google Scholor - Sadr-Azodi O, Konings P, Brusselaers N. (2017). Menopausal hormone therapy and pancreatic cancer risk in women: a population-based matched cohort study. United European Gastroenterol J., 1123-1128.
Publisher | Google Scholor - Yadav D, Muddana V, O’Connell M. (2011). Hospitalizations for chronic pancreatitis in allegheny county, pennsylvania, USA. Pancreatology, 11:546-552.
Publisher | Google Scholor - Yang AL, Vadhavkar S, Singh G, et al. (2008). Epidemiology of alcohol-related liver and pancreatic disease in the United States. Arch Intern Med.168:649–656.
Publisher | Google Scholor - Silverman, Debra T.; Hoover, Robert N.; Brown, Linda M.; Swanson, G. Mari; Schiffman, Mark; et al. (2003) Why Do Black Americans Have a Higher Risk of Pancreatic Cancer than White Americans? Epidemiology, 14(1):45-54.
Publisher | Google Scholor - Kovi J, Heshmat MY. (1973). Incidence of cancer in Negroes in Washington, DC and selected African cities. Am J Epidemiol, 96: 401-413.
Publisher | Google Scholor - Hao Y, Ward EM, Jemal A, Pickle LW, Thun MJ. (2006). U.S. congressional district cancer death rates. Int J Health Geogr., 5:28.
Publisher | Google Scholor