Case Report
The Skeletal Muscle of Humans is Affected by Testosterone through Cellular and Molecular Pathways-An Excuse to Increase Performances Illegally
Apollo college of pharmacy, anjora durg c.g, India.
*Corresponding Author: Hari Prasad Sonwani,Apollo college of pharmacy, anjora durg c.g, India.
Citation: Hari P. Sonwani. (2024). The Skeletal Muscle of Humans is Affected by Testosterone through Cellular and Molecular Pathways-An Excuse to Increase Performances Illegally. International Journal of Medical Case Reports and Reviews, BioRes Scientia Publishers. 3(3):1-9. DOI: 10.59657/2837-8172.brs.24.047
Copyright: © 2024 Hari Prasad Sonwani, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: February 28, 2024 | Accepted: March 30, 2024 | Published: April 09, 2024
Abstract
Because testosterone has such strong impacts on muscular mass and strength, it is very popular among drug users. Significant mechanisms underlying the mycotrophic effects of testosterone have been discovered in both short-term controlled trials and athletes who have been using steroids for years. By increasing protein synthesis, both short-term and long-term steroid use highlight the extent of fiber hypertrophy in human skeletal muscle. Activating satellite cells and promoting myonuclear accretion when preexisting myonuclear are no longer able to sustain further improvement of protein synthesis is one way that testosterone aids in the hypertrophy of muscle fibers. It's interesting to note that long-term steroid use also increases the frequency of fibers with myonuclear positioned in the center, suggesting the presence of strong regeneration activity. When testosterone is present, some daughters Proliferating satellite cells have the ability to elude differentiation and revert to quiescence, so contributing to the replenishment of the satellite cell reserve pool. It is unknown, therefore, if prolonged steroid use has a negative impact on satellite cells. Additionally, testosterone may promote pluripotent precursor cell commitment to myotubes while suppressing adaptogenic differentiation. It's believed that androgen receptors expressed in myonuclear and satellite cells mediate the effects of testosterone on skeletal muscle. There may possibly be an androgen-receptor-independent route, according to some data. It is evident that excessive testosterone use is linked to a strong activation of several myogenic pathways. This gives them an unfair edge over others who don't utilize drugs. It is unknown what the long-term effects will be on skeletal muscle's ability to regenerate.
Keywords: satellite cells; anabolic steroids; exercise training; androgen receptor; hypertrophy; hyperplasia; muscle fiber; regeneration; myonuclear; protein synthesis
Introduction
Many professional athletes, amateurs, school-age children, and a sizable portion of the general public who just want to look better take testosterone and similar steroids. The potent effects of testosterone and similar steroids on muscle masss and strength account for their popularity among drug users. Important cellular and molecular mechanisms underlying the mycotrophic action of anabolic steroids have been revealed by recent research. There are various myogenic routes by which the effects of testosterone may be mediated. This review begins with a brief explanation of the rationale behind testosterone use and the techniques employed to identify abuse of the hormone. The description of the cellular and structural modifications seen in human skeletal muscle as a result of administration of testosterone. We go over key physiological and molecular mechanisms that testosterone may use to affect skeletal muscle. The 19-carbon steroid testosterone has strong anabolic and androgenic properties. The Leydig cells in the testes are the main source of testosterone production; adrenal cortex and peripheral androstenedione conversion also contribute a tiny amount. The rationale for the appeal of testosterone and similar steroids among drug users is their anabolic action on skeletal muscle. Oral, intramuscular, and topical administration are the three methods of taking anabolic androgenic steroids. According to Yesalis (1993) and Hartgens and Kuipers (2004), these medications are used to improve attractiveness, increase lean body mass, decrease fat mass, improve performance, and survive intense training periods. (Yesalis, 1993; Wilson, 1988; Hartgens and Kuipers, 2004). In this regard, strong testosterone treatments for human neuroblastoma cells reduce cell viability by Identifying the misuse of testosterone ADA (2004); WADA (2004). The reason for this is because exogenous chemicals have a lower 13C content than their endogenous counterparts (Aguilera et al., 2001; Shakleton et al., 1997). In the event where a T/E44.0 is obtained but GC-C-IRMS is unable to definitively indicate exogenous delivery, longitudinal monitoring of the T/E-time profile becomes necessary. According to Sottas et al. (2007), the T/E ratio has greater inter-individual variability than intra-individual variability in this regard. It is also possible to find a T/E ratio in the 4:1 range among people who do not take testosterone. Consequently, a twofold increase in the T/E ratio could go unnoticed. It is proposed that a population-based T/E reference is insensitive to individual differences because of this. Sottas et al. (2007) state that the issue can.
Mass, strength, and testosterone in muscles
The benefits of testosterone for muscular growth and athletic performance are a major factor in its appeal to drug users. Gonadotropin-releasing hormone (GnRH) analogue-induced suppression of endogenous testosterone production in young males led to significant reductions in rates of fat burning, muscle strength, and whole-body protein synthesis, as well as an increase in adiposity (Mauras et al., 1998). A favorable correlation was seen between the concentration of testosterone and fat-free mass, muscle size, and strength when the circulating testosterone levels were manipulated through concurrent treatment with GnRH analogues and exogenous testosterone (Bhasin et al., 2001). Additionally, a double-blind, randomized, placebo-controlled experiment revealed that the body's reaction to a 12-week A set of patients receives a GnRH analogue once every fourth week, which attenuates strength training (Kvorning et al., 2006). Vorning and associates, 2006. Furthermore, several members of the myogenic regulatory factors (MyoD and Myogenin), insulin-like growth factor-1 (IGF-1), myostatin, and androgen receptors (ARs) do not appear to have their mRNA expression blunted by testosterone suppression (Kvorning et al., 2007). This clarifies the intricate control of the signaling pathways behind the growth of skeletal muscle in humans as a result of resistance training. Friedl and colleagues, 1991). Similar to this, both trained and untrained men's muscle strength and quadriceps cross-sectional area significantly increased after receiving supraphysiological dosages of testosterone (600 mg week—1) for 10 weeks (Bhasin et al., 1996). Additionally, it was demonstrated that short-term changes in upper body strength and body composition were produced by modest dosages of testosterone combined with weight exercise (Giorgi et al., 1999). Additionally, data point to a dose-dependent relationship between testosterone administration and human skeletal muscle hypertrophy (Bhasin et al., 2001). Remarkably, the application of Changes in muscle pinnation angle and potentially fascicle length has been linked to testosterone and intense resistance training (Blazevich and Giorgi, 2001).
Testosterone and muscle fiber hypertrophy
Key mechanisms underlying the potent myotrophic effects of testosterone were initially discovered in a group of elite powerlifters who reported using the drug for 9±3.3 years at a dose of 100–500 mg weekly (Kadiet al., 1999; Kadi, 2000). In powerlifters who are already well-trained, long-term testosterone administration enhances the degree of fiber hypertrophy (Kadi et al., 1999; Kadi, 2000). Both type I and type II muscle fibers enlarge when exposed to testosterone. The biggest muscle fibers in powerlifters, whether they use steroids or not, are type II muscle fibers. Nevertheless, data indicates that slow type I muscle fibers show the most variation in growth between steroid users and non-users. (Eriksson et al., 2005; Kadi et al., 1999; Kadi, 2000). The area of type I muscle fibers in the trapezius muscle of steroid users is 58% bigger than that of non-users, whereas the area of type II muscle fibers is 33% larger (Kadi et al., 1999). According to Eriksson et al. (2005), the vastus lateralis has the similar tendency. It follows that type I muscle fibers have been demonstrated to be more responsive to anabolic drugs than type II muscle fibers (Hartgens et al., 1996). Later research revealed that whereas type II muscle fibers only enlarge following the administration of 600 mg testosterone, type I muscle fiber area increased in response to 300 and 600 mg of testosterone (Sinha-Hikim et al.)
Myonuclear content, protein synthesis, and testosterone
One key way that testosterone can increase muscle fiber size is through increased synthesis of contractile proteins. When 200 mg of testosterone enanthate was injected intramuscularly into healthy adults, net protein synthesis increased significantly by twofold while protein breakdown remained constant (Ferrando et al., 1998). Testosterone improved the skeletal muscle's repurposing of intracellular amino acids but had no effect on the inward transport of amino acids to the muscle (Ferrando et al., 1998). Known as "nuclear domains," adult muscle fibers are made up of hundreds of myonuclei, each of which maintains protein synthesis over a limited amount of cytoplasm (Cheek, 1985). In this regard, a notable increase in muscle fiber enlargement (36% increase in fiber cross-sectional area) is correlated with a notable rise in (Kadi et al., 2004b, 2005; Kadi, 2000). Thus, it is proposed that a certain amount of fiber hypertrophy can be supported by the myonuclei that now exist. However, the increase in myonuclei number is believed to become engaged in the enhancement of protein synthesis when the transcriptional activity of already-existing myonuclei reaches its maximum—a concept known as the ceiling theory (Kadi et al., 2004b, 2005; Petrella et al., 2006). The correlation between the number of myonuclei per muscle fiber cross-section and the cross-sectional area of the fibers provides more evidence for this (Kadi, 2000; Kadi et al., 2006). In this regard, myonuclear accretion is one way that testosterone promotes the hypertrophic of muscle fibers observed in drug users (Kadi et al., 1999; et al., Sinha-Hikim (2002). Myonuclear accretion is higher in type I muscle fibers (+23%) compared to type II muscle fibers (+14%) in high-level powerlifters, and the mean number of myonuclei per fiber cross-section is significantly larger in steroid users compared with non-users (Kadi, 2000). This makes sense given that those who use steroids tend to have higher hypertrophy of type I muscle fibers.
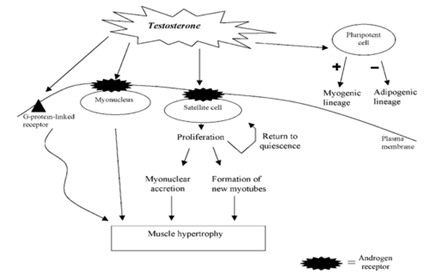
Figure 1: Mechanisms of testosterone action on skeletal muscle.
Testosterone and centrally located myonuclei
The percentage of muscular fibers with internal myonuclei in the vastus lateralis and the trapezius muscle in powerlifters who use steroids reaches 25% and 29%, respectively (Kadi et al., 1999; Eriksson et al., 2005). The percentage of fibers with internal myonuclei in the vastus lateralis and trapezius of powerlifters who do not use steroids is 9% and 5%, respectively (Kadi et al., 1999; Eriksson et al., 2005). This suggests that centrally placed myonuclei in the vastus lateralis and trapezius are increased by three to five times in response to testosterone. Centrally positioned myonuclei are found in both type I and type II muscle fibers in steroid users. On the other hand, type II muscle fibers include the majority of centrally positioned myonuclei in non-steroid users (Kadi et al., 1999). Conventionally, the existence of internal myonuclei is acknowledged as proof that muscle regeneration is still occurring. When satellite cells are activated, they may proliferate and differentiate into new myotubes, which may eventually fuse with the muscle fibers that already exist. Some myonuclei may become caught in the center of the newly formed, bigger fiber during the fusion process. The myonuclei's physiological location may be necessary for fiber growth when newly formed myotubes connect with preexisting parent muscle fibers. After fusion, centrally placed myonuclei may hold onto their positions for a while, which would shorten the diffusion distance between a nucleus and the center of the myofibre.
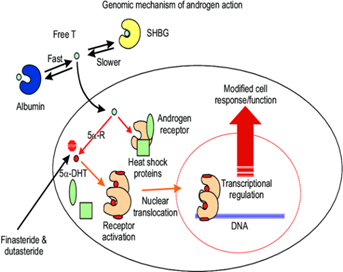
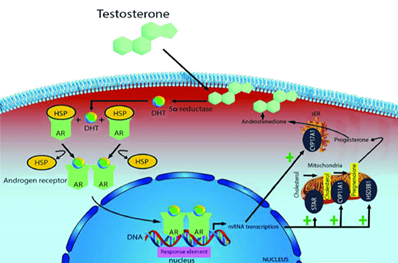
Figure 2: Universal genomic molecular mechanism of androgen action. One key biochemical reaction in this sequence of pathways is the transformation of testosterone (T) to 5α-dihydrotestosterone (5α-DHT) via 5α-reductases (5α-Rs). This pathway is critical in the function of androgen receptor, since 5α-DHT is more potent natural ligand than T. SHBG: sex hormone binding globulin.
Testosterone and satellite cells
Between the muscle fibers' basal lamina and plasma membrane are satellite cells (Mauro, 1961).2005; Kadi et al., 2004a). Type I and type II muscle fibers in the human vastus lateralis of young adults have the same amount of satellite cells per fiber cross-section (Kadi et al., 2006). Adult muscle fibers have post-mitotic myonuclei, and the primary source of additional myonuclei added to the hypertrophying muscle fiber is muscle satellite cells (Moss and Leblond, 1971). The activation and proliferation of satellite cells may be regulated by a range of changes in their external milieu, such as mechanical and growth factors as well as hormonal signaling (Kadi, 2005; Kadi et al., 2005; Mackey et al., 2007). satellite-produced cells Testosterone and centrally positioned myonuclei, although no changes in the number of myonuclei are seen when the increase in fiber area is not greater than about 26% (Kadi et al., 2005; Kadi, 2000). Currently, it is unknown what processes govern the destiny of daughter cells produced by activating satellite cells. In myoblast culture systems, testosterone can induce satellite cells to undergo mitosis (Powers and Florini, 1975). Furthermore, human skeletal muscle shows an increase in PCNA + (proliferating cell nuclear antigen-positive) satellite cells in response to testosterone therapy. The fact that PCNA-expressing satellite cells exist suggests that testosterone may facilitate the entry of satellite cells into the cell cycle (Hikim and Sinha, 2006). This emphasizes how testosterone's myotrophic effect on skeletal muscle is mediated by satellite cells. Some daughter cells can evade differentiation and return to quiescence when driven into the cell cycle by satellite cells, which ultimately results in the production of new satellite cells. In this regard, a notable rise in the quantity of satellite cells has been documented in males given testosterone for 20 weeks at doses of 300 and 600 mg week—1 (Sinha-Hikim et al., 2003). The quantity of satellite cells in the power skeletal muscle Lifters who have been on anabolic steroids for 9±3.3 years are more than in young, healthy guys, but it's still comparable to what's observed in powerlifters who don't use steroids (Kadi, 2000). One theory is that short-term steroid administration promotes the development of new satellite cells, while long-term users may not experience this primary outcome for their newly formed daughter cells. On the other hand, long-term steroid use reduces the effects of the drug on satellite cells. Skeletal muscle stem cells are known as muscle satellite cells. Therefore, they are able to keep a ready supply of precursors for muscle fibers. Skeletal muscle satellite cell self-renewal is explained by a number of different ways. One or two daughter cells from an asymmetric or symmetric cell division would develop into additional satellite cells. A transmembrane receptor called Notch has the ability to activate transcription factors that control cell destiny. Asymmetric cell divisions have been suggested to be caused by the asymmetric expression of Numb (membrane-associated inhibitor of the Notch signaling) in dividing satellite cells in vitro (Conboy and Rando, 2002). While Numb-negative cells would elude development and turn into satellite cells, Numb-positive cells would proceed into differentiation. According to Conboy and Rando (2002), there is a theory that Numb-negative cells will keep multiplying and replenish the satellite cell pool. Thus, one route that may be implicated in regulating the destiny of freshly produced cells is the interplay between Notch and its antagonist Numb. Accordingly, it is hypothesized that testosterone may affect the destiny of freshly formed daughter cells by upregulating the expression of the activated version of Notch, which would encourage cell division and the production of fresh satellite cells (Sinha-Hikim et al.)
Testosterone and the commitment of pluripotent precursor cells into myogenic lineage
It is unknown, therefore, what role these muscle precursor cells play in the supraphysiological and physiological adaptations of human skeletal muscle. However, it has been demonstrated that when treated with testosterone, mouse C3H 10T1/2 pluripotent mesenchymal cells—which are cells that can differentiate into muscle, fat, cartilage, and bone cells—begin to exhibit particular myogenic markers (MyoD and myosin heavy chains) (Singh et al., 2003).
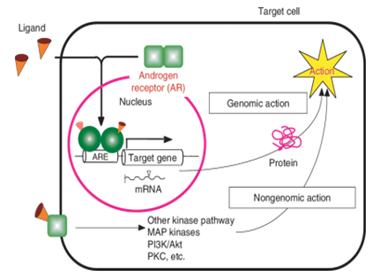
Figure 3: Biological actions of androgens via ARs. Androgen binds to the AR. The liganded AR forms homodimers and moves it into the nucleus to bind to specific DNA elements referred to androgen-responsive elements in target gene promoters and regulates the target gene expression at the transcriptional level. This action of the AR is called genomic action. Androgen-AR also exerts its function without transcription by interaction with other signaling pathways. This action is called nongenomic action.
Testosterone and the differentiation of adipocytes
Because it is a strong regulator of lipolysis and affects catecholamine signal transduction in fat cells, testosterone can also help reduce body fat (Arner, 2005). According to certain theories, testosterone promotes lipolysis and prevents adipocytes from absorbing fat (De Pergola, 2000). Similarly, data point to testosterone's potential to prevent adipocyte precursor cell differentiation (De Pergola, 2000). According to these findings, adipocyte counting and the expression of two adipogenic inhibitory factors (PPARd2 (peroxisomal proliferator-activated receptor) and CCAAT/enhancer-binding protein a)) show that treating mouse C3H 10T1/2 pluripotent cells with testosterone inhibits adipogenic differentiation (Singh et al., 2003).
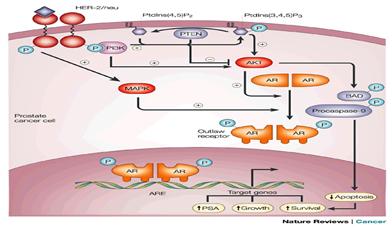
Figure 4: How growth factor signal transduction creates outlaw receptors.In the tumour cells of a patient receiving androgen ablation therapy, HER-2/neu (and possibly other receptor tyrosine kinases) can become overexpressed. HER-2/neu indirectly activates mitogen-activated protein kinase (MAPK). MAPK might phosphorylate the androgen receptor (AR), creating an androgen-independent 'outlaw' receptor. An alternative means by which HER-2/neu (or other pathways) might activate the AR is by activating the AKT (protein kinase B) pathway. In this pathway, activation of receptor tyrosine kinases, such as HER-2/neu, increase the level of phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5) P3) by activating phosphatidylinositol 3-kinase (PI3K). Another pathway might involve inactivation of the lipid phosphatase PTEN, so that PtdIns(3,4,5)P3 can no longer be converted back to its substrate, PtdIns(4,5)P2. AKT is activated by PtdIns(3,4,5)P3, and might be able to produce an outlaw AR by phosphorylating it. AKT can also activate parallel survival pathways by phosphorylating and inactivating pro-apoptotic molecules such as BAD and procaspase-9. ARE, androgen response element; PSA, prostate-specific antigen.
Androgen receptors: intermediaries of the actions of testosterone
Multiple myogenic pathways work together in a complicated manner to control the process of muscle fiber hypertrophy (Figure 1). In this way, the hypertrophy of rat muscle fibers was inhibited by the blockage of ARs by the AR antagonist oxendolone (Inoue et al., 1994). The role that ARs may play as potential mediators of the exercise-induced hypertrophy of muscle fibers is amply demonstrated by this investigation. Studies also showed significant increases in AR concentration in response to physical activity (Deschenes et al., 1994; Bamman et al., 2001). The super family of ligand-responsive transcription regulators includes ARs. The androgen-receptor complex is translocated to the nucleus' hormone-responsive element after androgenic hormones bind to the receptor, activating it. The connection (Coffey and Luke, 1994). Rat prostate tissue has many times more binding sites per milligram of protein than skeletal muscle (Krieg, 1976). In the myonuclei of human muscle fibers, two investigations (Ruizeveld de Winter et al., 1991; Janssen et al., 1994) were unable to detect a positive immunostaining. The low amount of AR in skeletal muscle is most likely the cause of the lack of staining when utilizing traditional immunohistochemical techniques. On the other hand, human vastus lateralis and trapezius muscles exhibit positive immunolabeling when immunohistochemistry is carried out using a signal amplifi-cation approach (Kadi, 2000; Kadi et al., 2000). Both intramuscular nerve bundles and capillary endothelium exhibit immunolabeling (Kadi et al., 2000). Interestingly, not all muscles and myonuclei express AR. The measurement of AR-containing myonuclei in each fiber cross-section demonstrated notable variations between the two muscles: The percentage of AR-containing myonuclei in the trapezius in untrained participants compared to the vastus lateralis, was over 60% greater (Kadi et al., 2000). Data indicating that androgen sensitivity may differ amongst distinct muscle groups (Kochakian and Tillotson, 1957; Wilson, 1988) corroborate these findings. It is possible to change the percentage of AR-containing myonuclei in human skeletal muscle by self-administering androgenic–anabolic drugs. The percentage of AR-positive myonuclei and the myonuclear number in the trapezius of athletes who take steroids are higher than those of non-users. Athletes who use steroids have more myonuclei in their vastus lateralis, but their percentage of myonuclei that contain AR is comparable to that of athletes who do not use steroids. According to certain theories, the treatment paradigm may cause the expression of AR to stabilize (Ferrando et al., 2002). The intricacy of AR control in human skeletal muscle under physiological and supraphysiological circumstances is demonstrated by these findings taken together.
Androgen receptors are expressed by satellite cells
The immunological finding of AR in pig myogenic satellite cells in vitro (Doumit et al., 1996) provides evidence for the impact of testosterone on satellite cells (Figure 1). Additionally, therapy with testosterone resulted in an increase in immunoreactive AR (Doumit et al., 1996). As a result, testosterone directly targets satellite cells. Human muscle cells later showed comparable outcomes (Sinha-Hikim et al., 2004). The augmentation of androgen-binding sites may play a role in modulating pathways that govern satellite cell function.
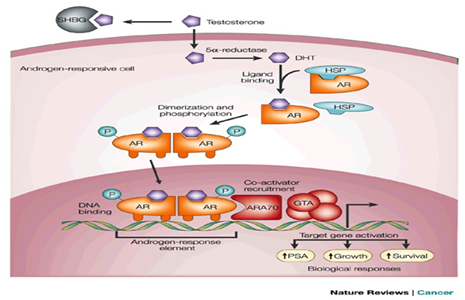
Figure 5: Androgen action. Testosterone circulates in the blood bound to albumin (not shown) and sex-hormone-binding globulin (SHBG), and exchanges with free testosterone. Free testosterone enters prostate cells and is converted to dihydrotestosterone (DHT) by the enzyme 5-reductase. Binding of DHT to the androgen receptor (AR) induces dissociation from heat-shock proteins (HSPs) and receptor phosphorylation. The AR dimerizes and can bind to androgen-response elements in the promoter regions of target genes6. Co-activators (such as ARA70) and corepressors (not shown) also bind the AR complex, facilitating or preventing, respectively, its interaction with the general transcription apparatus (GTA). Activation (or repression) of target genes leads to biological responses including growth, survival and the production of prostate-specific antigen (PSA). Potential transcription-independent actions of androgens are not shown.
The androgen-receptor-independent pathway: intermediaries of the effects of testosterone
According to recent research, testosterone may have a quick intracellular AR-independent route of action (Figure 1). The activation of a G-protein-linked receptor at the plasma membrane of myoblasts isolated from rat newborn hind limbs may be the mechanism by which this quick non-genomic testosterone effect is achieved (Estrada et al., 2003). This would cause an extracellular signal-regulated kinase (ERK1/2) to become active early but briefly activation, which may cause transcription factors linked to cellular development to become phosphorylated (Estrada et al., 2003). The physiological significance of testosterone's fast non-genomic effect in human skeletal muscle is still unknown.
Figure 6: The skeletal muscle of humans is affected by testosterone through cellular and molecular pathways. An excuse to increase performances illegally
Conclusion
A strong effect of testosterone is seen in human skeletal muscle. Research on the effects of testosterone on the muscles makes it abundantly evident that abusing drugs is linked to a strong activation of several myogenic pathways. Testosterone administration in sports undoubtedly gives athletes a distinct physical advantage over non-users. It is unknown what effects the massive recruitment of satellite cells will have in the long run on the skeletal muscle's ability to regenerate itself and their proliferative capability.
Abbreviations
AR-androgen receptor;
GnRH-gonadotropin-releasing hormone;
PCNA-proliferating cell nuclear antigen;
T/Erato-testosterone/epitestosterone ratio.
References
- Mauro A (1961). Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol, 9:493-495.
Publisher | Google Scholor - Moss FP, Leblond CP (1971). Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec, 170:421-435.
Publisher | Google Scholor - Petrella J, Kim J, Cross J, Kosek D, Bamman M (2006). Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab, 291:937-946.
Publisher | Google Scholor - Powers ML, Florini JR (1975). A direct effect of testosterone on muscle cells in tissue culture. Endocrinology, 97:1043-1047.
Publisher | Google Scholor - Ruizeveld de Winter JA, Trapman J, Vermey M, Mulder E, Zegers ND, van der Kwast TH (1991). Androgen receptor expression in human tissues: an immunohistochemical study. J Histochem Cytochem, 39:927-936.
Publisher | Google Scholor - Shackleton CH, Phillips A, Chang T, Li Y (1997). Confirming testosterone administration by isotope ratio mass spectrometric analysis of urinary androstanediols. Steroids, 62:379-387.
Publisher | Google Scholor - Singh R, Artaza J, Taylor W, Gonzalez-Cadavid N, Bhasin S (2003). Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology, 144:5081-5088.
Publisher | Google Scholor - Sinha-Hikim I, Artaza J, Woodhouse L, Gonzalez-Cadavid N, Singh AB, Lee MI et al. (2002). Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol, 283:154-164.
Publisher | Google Scholor - Sinha-Hikim I, Cornford M, Gaytan H, Lee ML, Bhasin S (2006). Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J Clin Endocrinol Metab, 91:3024-3033.
Publisher | Google Scholor - Sinha-Hikim I, Roth SM, Lee MI, Bhasin S (2003). Testosterone- induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. Am J Physiol, 285:197-205.
Publisher | Google Scholor - Sinha-Hikim I, Taylor WE, Gonzalez-Cadavid NF, Zheng W, Bhasin S (2004). Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J Clin Endocrinol Metab, 89:5245-5255.
Publisher | Google Scholor - Sottas PE, Saudan C, Schweizer C, Baume N, Mangin P, Saugy M (2008). From population- to subject-based limits of T/E ratio to detect testosterone abuse in elite sports. Forensic Sci Int, 174:166–172.
Publisher | Google Scholor - WADA (2004). Technical document: reporting and evaluation guidance for testosterone, epitestosterone, T/E ratio and other endogenous steroids. Wilson JD (1988). Androgen abuse by athletes. Endocr Rev, 9:181-199.
Publisher | Google Scholor - Yesalis CE (ed). (1993). Incidence of anabolic steroid use: a discussion of methodological issues. Anabolic Steroids in Sport and Exercise. Human kinetics: champaign, IL, USA, 49-69.
Publisher | Google Scholor - Janssen PJ, Brinkmann AO, Boersma WJ, Van der Kwast TH (1994). Immunohistochemical detection of the androgen receptor with monoclonal antibody F39.4 in routinely processed, paraffin- embedded human tissues after microwave pre-treatment. J Histochem Cytochem, 42:1169-1175.
Publisher | Google Scholor - Kadi F (2000). Adaptation of human skeletal muscle to training and anabolic steroids. Acta Physiol Scand, 646:1-52.
Publisher | Google Scholor - Kadi F (2005). Hormonal and growth factor-related mechanisms involved in the adaptation of skeletal muscle to exercise. In: Kraemer W, Rogol A (eds). The endocrine System in Sports and Exercise. Olympic Encyclopaedia of Sports Medicine vol. XI, Chapter 22. Blackwell: Massachusetts, USA, 306-318.
Publisher | Google Scholor - Kadi F, Bonnerud P, Eriksson A, Thornell LE (2000). The expression of androgen receptors in human neck and limb muscles: effects of training and self-administration of androgenic–anabolic steroids. Histochem Cell Biol, 113:25-29.
Publisher | Google Scholor - Kadi F, Charifi N, Denis C, Lexell J (2004a). Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve, 29:120-127.
Publisher | Google Scholor - Kadi F, Charifi N, Denis C, Lexell J, Andersen JL, Schjerling P et al. (2005). The behaviour of satellite cells in response to exercise: what have we learned from human studies? Pflugers Arch, 451:319-327.
Publisher | Google Scholor - Kadi F, Charifi N, Henriksson J (2006). The number of satellite cells in slow and fast fibres from human vastus lateralis muscle. Histochem Cell Biol, 126:83-87.
Publisher | Google Scholor - Kadi F, Eriksson A, Holmner S, Thornell L-E (1999). Effects of anabolic steroids on the muscle cells of strength-trained athletes. Med Sci Sports Exerc, 31:1528-1534.
Publisher | Google Scholor - Kadi F, Schjerling P, Andersen LL, Charifi N, Madsen JL, Christensen LR et al. (2004b). The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol, 558:1005-1012.
Publisher | Google Scholor - Kochakian CD, Tillotson C (1957). Influence of several C19-steroids on the growth of individual muscles of the guinea pig. Endocrinol- ogy, 60:607-618.
Publisher | Google Scholor - Krieg M (1976). Characterization of the androgen receptor in the skeletal muscle of the rat. Steroids, 28:261-274.
Publisher | Google Scholor - Kvorning T, Andersen M, Brixen K, Madsen K (2006). Suppression of endogenous testosterone production attenuates the response to strength training: a randomized, placebo-controlled, and blinded intervention study. Am J Physiol, 291:1325-1332.
Publisher | Google Scholor - Kvorning T, Andersen M, Brixen K, Schjerling P, Suetta C, Madsen K (2007). Suppression of testosterone does not blunt mRNA expres- sion of myoD, myogenin, IGF, myostatin or androgen receptor post strength training in humans. J Physiol, 578:579-593.
Publisher | Google Scholor - Luke M, Coffey D (1994). The male accessory tissues: structure, androgen, and physiology. In: Knobil E, Neill J (eds). The Physiology of the Reproduction. Raven Press: New York,1435-1488.
Publisher | Google Scholor - Mackey AL, Kjaer M, Dandanell Jorgensen S, Mikkelsen KH, Holm L, Dossing S et al. (2007). The influence of anti-inflammatory medication on exercise-induced myogenic precursor cell responses in humans. J Appl Physiol, 103:425-431.
Publisher | Google Scholor - Mauras N, Hayes V, Welch S, Rini A, Helgeson K, Dokler M et al. (1998). Testosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metab, 83:1886-1892.
Publisher | Google Scholor