Research Article
The Role of Maternal Microchimeric Cells in Cancer Development
1Department of Medical Biology, Faculty of Medicine, Çukurova University, Balcali- Adana/Turkey.
2Department of Medical Biology, Faculty of Medicine, Adıyaman University, Adıyaman, Turkey.
*Corresponding Author: Osman Demirhan, Department of Medical Biology, Faculty of Medicine, Çukurova University, Balcali- Adana/Turkey.
Citation: Osman Demirhan, Deniz Tastemir Korkmaz, Nesrin Cetine Senturk. (2023). The Role of Maternal Microchimeric Cells in Cancer Development, Journal of Cancer Management and Research, BRS Publishers. 1(1):1-7. DOI: 10.59657/2996-4563.brs.23.002
Copyright: © 2023 Osman Demirhan, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: March 03, 2023 | Accepted: March 25, 2023 | Published: March 31, 2023
Abstract
Research question: Physiological or pathological roles of microchimeric cells (McCs) are not fully known and no definite information has been revealed yet. Revealing the histopathology of fetal-maternal microchimeric cells (F-MMcCs) may be an indicator of the future evolution of diseases, especially tumors, and may create new models.
Design: The presence of McCs in cancer tissue has been evaluated using FISH and cytogenetic techniques.
Results:We found 18% maternal microchimeric cells (MMcCs) in tumor tissue cells and 0.2% in peripheral blood cells of the patient with UPS. The frequency of these cells in tumor tissue was much higher than in blood tissue (p<0.0001).
Conclusion: Current findings reveal the current status and role of feto-maternal microchimerism (F-MMc) in cancer tissues and may provide a new perspective on Mc. Mc is a physiologicalphenomenon. However, microchimeric cells (McCs) can turn into pathology under unsuitable conditions and become a multistage part ofthe pathogenesis.
Keywords: microchimerism; fetal-maternal microchimeric cells; pleomorphic sarcoma
Introduction
Mc is the presence of small amounts of foreign cells in an individual's circulation or tissues. Mc can occur naturally and artificially in humans. The natural Mc is stem cell exchange between mother and fetus during pregnancy, which can lead to the existence of genetically unique cells that can persist for decades in both mother and child. This is the most common type of occurring human chimerism (Boddy et al, 2015). As a result of this; potentially Mc formation occurs in both mother and offspring. Thus, no one is born pure, so we are all born microchimeric (Mc). So, what are the physiological functions of these stem cells? The biological implications of McCs are still largely unexplored. Therefore, there are many unanswered questions about chimerism. It has been suggested to have pathogenic, beneficial and neutral roles for FMcCs (Galofré 2012). How these cells survive, how they adapt to the new environment, and how they acquire their ability to differentiate has not yet been explained. While their function is not yet known, it is crucial to functionally understand what McCs do in a normal healthy pregnancy and postpartum or offspring. Related to this, the clinical effects of MMcCs in organ repair, cancer development and treatment are only just beginning to be understood. In this study, we will try to explain the presence and potential effects of McCs in tumor tissues.
Materials and Methods
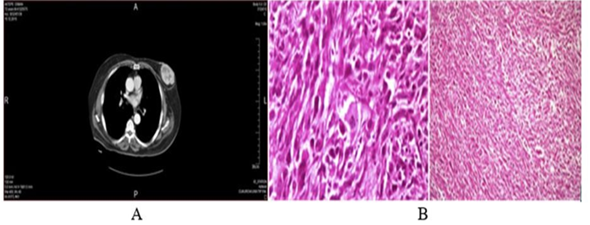
In this study, a 73-year-old male patient with a sarcoma who had an enlarging mass in his left breast was examined. There was no history of trauma and nipple discharge or other breast lumps. The patient had no history of breast cancer in the past. In addition, she had never received chemotherapy or radiotherapy and had no history of blood transfusions. The patient's breast ultrasound image showed a solid, hypoechoic mass with central necrosis in the left breast. The right breast and right armpit were normal. However, computed tomographic analysis revealed a 5x8cm mass with high peripheral vascularity and a hypodense necrotic center.
Figure A: A computed tomography scans of the mass: Normal bilateral axilla the tumour mass in the left breast with high peripheral vascularity and hypodense necrotic centre.
Figure B: pathological images of undifferentiated pleomorphic sarcoma.
Fluorescence In Situ Hybridization (FISH)
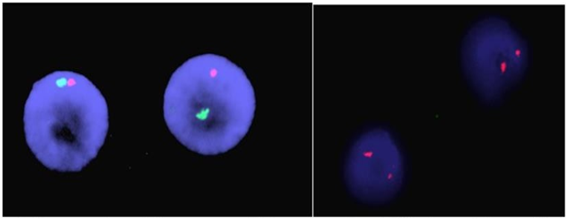
Tissue samples of the primary tumor were taken by doctors in the general surgery department. Tumor tissue piece and 3-5 ml peripheral blood sample were taken for genetic studies. FISH and chromosome analyze of cancer and blood samples were performed in the genetics laboratory of Çukurova University, Faculty of Medicine, Department of Medical Biology. Tumor samples were cut into pieces mechanically and enzymatically dissociated with trypsin-EDTA (Biological Industries Israel Beit-Haemek Ltd.) for 1 hour. Standard cytogenetic techniques were used for interphase FISH. For this purpose, the method of Taştemir Korkmaz et al. was used (Taştemir Korkmaz et al, 2021). CEP X Spectrum Orange/Y Spectrum Green DNA Probe Kit (Abbott) was used to detect numerical abnormalities of X and Y chromosomes in tumor cells. A Y chromosome (green signal) and an X chromosome (red signal) signal were reported in the nuclei of male cells. Two X signals were found in cells of female origin.
Figure 2: Copy number of chromosomes Y (green spots) and X (red spots) detected by fish in sarcoma cancer cells from a man case, two tumor nucleus showing XX chromosomes.
Approximately 500 interphase cells were analyzed using a BX51 Olympus fluorescence microscope equipped with Cytovision Probe Software (Applied Imaging, Santa Clara, CA).
Results
The findings indicating the presence of fetal and maternal cells in the blood or tissues of cancer patients are shown in Table 1. The presence of McCs in cancer patients has been evaluated using FISH and cytogenetic techniques. We found 18% MMcCs in tumor tissue cells and 0.2% in peripheral blood cells of the patient with UPS analyzed by FISH. The frequency of these cells in tumor tissue was much higher than in blood tissue (p<0>
Table 1: Microchimeric cells in the blood and malignant tissue of the patient.
| Microchimeric cells (Number of cells with anomaly/total number of cells analyzed) (%) | ||||
| Case No | Age/Sex | Tumor type | Blood | Tissue |
| Pleomorphic Sarcoma (PS) | ||||
| 1 | 73/M | PS | 1/528 (0.2%) | 95/528 (18%) |
Discussion
Today, we are only beginning to understand that McCs may play a role in becoming cancerous. Because there are so many unknowns about it. For example, it is still unknown whether Mc can be involved in the carcinogenetic process or whether fetal micr ochimeric cells (FMcCs) can differentiate into host tissues and participate in the maternal response to injury. Intense research has been carried out in recent years to clarify controversial reports and uncertainties on this subject. In the present study, our current findings and related literature are reviewed, trying to explain the relationship of McCs with cancer and their functions in tissues. In the present study, 18% of tumor tissue cells and 0.2% of peripheral blood tissue cells of patients with UPS were found to be composed of MMcCs. The frequency of these cells in tumor tissue was much higher than in blood tissue (p<0>The presence of FCs has been associated with both positive and negative effects on maternal health. Recently, many researchers have shown that cancer has the potential to be affected by McCs. It is thought that fetal mesenchymal progenitors and leukocytes may harbortumors after fetal progenitor cells are found in the inflammatory skin of the mother. The prevalence of McCs in cancer- affected patients was found to be significantly lower compared to healthy controls. It has been suggested that this may be due to increased clearance of FCs and specific uptake of FCs by an activated maternal immune system. FMc has been associated with some classical autoimmune diseases, but the role of these cells in normal health has not been defined. Following pregnancy, FCs may cause a graft-host-like reaction in women, and the maternal immune response against these foreign cells may support an autoimmune reaction.A potential function of these cells may be in the surveillance of malignant cells. To assess the presence and potential role of FCs in maternal cancers, the researchers examined maternal bloodor tumor tissue during or after pregnancy. The presence of FMcCs was found in cervical tissuesobtained from patients with cervical cancer (Cha et al, 2003). It has been reported that the levels of FCs in lungand thyroid tumors of women with a history of pregnancy are much higher than in the surrounding healthy tissue (O’Donoghue et al, 2008; Cirello et al, 2008). Perhaps the micro-environment of stem cells in normal tissues may serve in a similar way to transform FCs in the lung into cancer stem cells. On the other hand, besides systemic sclerosis, a few other diseases have been associated with Mc. An alternative carcinogenetic role of FMcCs has been suggested in melanoma and colon cancer developing during pregnancy (Kamper- Jørgensen et al, 2012). Some researchers have therefore suggested that Mc is one partin multistep pathogenesis. Considering the potential of FMcCs to differentiate into different cells and their persistence in women after pregnancy, these cells may also act as cancer stem cells and cause tumors as a result of genetic changes or changes in their microenvironmental niches. This idea will open new avenues for understanding tumor biology and stroma formation.Our findings may also provide a new perspective on sarcoma, lung, and bladder cancers. It hasbeen recently thought that FMc may play a role in tumor development in women who have given birth. However, the potential for FCs to develop into tumorigenesis-initiating cells in theinappropriate microenvironment, it seems plausible, but as yet unproven. At the same time, it suggests that FMcCs, which have also been identified in the supporting stroma tissue of the tumor, have deleterious roles. It is therefore possible that MMc might affect risk of some typesof cancer in women (and men). Just as it caused cancer in the breast area in our male patient. Evidence has also been suggested for maternal cancer cells that form tumors in the fetus (Potter and Schoeneman 1970; Brodsky et al, 1965; Holland 1994). On the other hand, the findings suggest that FMcCs may play a role in breast physiology, but the effects on maternal health are not yet clear. However, there is anecdotal evidence that MMcmay be involved in the penetration of some hereditary cancer syndromes and may form tumorsin the fetus (Cha et al, 2003; Cirello et al, 2008). At some time, an association between FCs in cancer stroma and cancer has been reported in women with pregnancy-associated invasive breast cancer (Dubernard et al, 2009). All these findings and information point out that McCs may also play a role in the development of cancer. Now, can we say more comfortable now that F-MMcCs cause cancer?
Conclusion
Although FMc's role in normal health has not been fully defined, it has been associated with several classic autoimmune diseases and a small number of cancers. The presence of McCs in tumors, normal and healing tissues has led to the fact that while it may play a protective role in some cancers, it may play a triggering role in some other cancers. Our results suggest that the presence of McCs in UPS tissue may play a role in carcinogenesis, further revealing the potential relationship between Mc and disease. Thus, some researchers have suggested that Mc may be part of the multistep pathogenesis. Actually, Mc is a physiological phenomenon, but McCs can turn into pathology under unsuitable conditions. While McCs sometimes act as immune surveillance and help suppress tumor growth, they can sometimes act as cancer stroma and contribute to the growth of tumors. Distinguishing and detecting McCs will help us understand the role of Mc in health and disease. It may also be indicative of the future evolution of the tumor and establish models.
Declarations
Acknowledgements: We thank the Doctors in Department of General Surgery, Department of Chest Diseases and Department of Urology, Faculty of Medicine, Cukurova University, Balcali- Adana, Turkey.
Informed Consent: It was obtained.
Author Contributions: Osman Demirhan: methodology, writing-review & editing, resources, project administration; Deniz Tastemir Korkmaz: project administration, review & editing; Nesrin Cetinel Senturk: methodology.
Ethical Committee Approval: The study was approved by Local Ethics Committee of the Cukurova University, Faculty of Medicine (approval number: TF2009LTP45 project).
Conflict of Interest: The authors declare no financial competing interests.
Financial Disclosure: The authors declared that this study received no financial support.
References
- Abat, D. Demirhan, O. Inandiklioglu, N. Tunc, E. Erdogan, et.al. (2014). Genetic alterations of chromosomes, p53 and p16 genes in low- and high-grade bladder cancer. Oncol Lett. 8(1):25-32.
Publisher | Google Scholor - Bianchi, D.W. Robert, E. Gross Lecture. (2007). Feto-maternal cell trafficking: a story that begins with prenatal diagnosis and may end with stem cell therapy. Journal of Pediatric Surgery. 42:12- 18.
Publisher | Google Scholor - Boddy, A.M. Fortunato, A. Sayres, M.W. Sayres, W. Aktipis, A.C. (2015). Fetal microchimerism and maternalhealth: A review and evolutionary analysis of cooperation and conflict beyond the womb. Bioessays. 37:1106-1118.
Publisher | Google Scholor - Brodsky, I. Baren, M., Kahn, S.B. Lewis, G. Tellem, J.M. (1965). Metastatic malignant melanoma from mother to fetus, Cancer. 18:1048–1054.
Publisher | Google Scholor - Cha, D. Khosrotehrani, K. Kim, Y. Stroh, H. Bianchi, et.al. (2003). Cervical cancer and microchimerism. Obstet. Gynecol. 102(4):774-781.
Publisher | Google Scholor - Cirello, V. Recalcati, M.P. Muzza, M. Rossi, S. Perrino, et.al. (2008). Fetal cell microchimerism in papillary thyroid cancer: a possible role in tumor damage and tissue repair. Cancer Res. 68(20):8482-8488.
Publisher | Google Scholor - Cirello, V. Perrino, M. Colombo, C. Muzza, M. Filopanti, et.al. (2010). Fetal cell microchimerism in papillary thyroid cancer: studies in peripheral blood and tissues. Int J Cancer. 126:2874-2878.
Publisher | Google Scholor - Cirello, V. Colombo, C. Perrino, M. De Leo, S. Muzza, M (2015). Fetal cell microchimerism in papillary thyroid cancer: A role in the outcome of the disease. Int J Cancer. 137(3-4):2989-2993.
Publisher | Google Scholor - Dadras, S.S. Paul, T. Bertoncini, J. Brown, L.F. Muzikansky, et.al. (2003). Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol. 162(6):1951-1960.
Publisher | Google Scholor - Dadras, S.S. Lange-Asschenfeldt, B. Velasco, P. Nguyen, L. Vora, et.al. (2005). Tumor lymph angiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod Pathol. 18(9):1232-1242.
Publisher | Google Scholor - Dawe1, G.S. Tan, X.W. Xiao, Z.C. (2007). Cell Migration from Baby to Mother. Cell Adhesion & Migration. 1(1):19-27.
Publisher | Google Scholor - Dhimolea, E. Denes, V. Lakk, M. Al-Bazzaz, S. Aziz-Zaman, et.al. (2013). High male chimerism in the female breast shows quantitative links with cancer. Int J Cancer. 133(4):835-842.
Publisher | Google Scholor - Dubernard, G. Aractingi, S. Oster, M. Rouzier, R. Mathieu, et.al. (2008). Breast cancer stroma frequently recruits fetal derived cells during pregnancy. Breast Cancer Res, 10:14.
Publisher | Google Scholor - Dubernard, G. Oster, M. Chareyre, F. Antoine, M. Rouzier, (2009). Increased fetal cell microchimerism in high grade breast carcinomas occurring during pregnancy. Int J Cancer. 124:1054-1059.
Publisher | Google Scholor - Eun, J.K. Guthrie, K.A. Zirpoli, G. Gadi, V.K. (2013). In situ breast cancer and microchimerism. Sci Rep. 3:2192.
Publisher | Google Scholor - Gadi, V.K. Nelson, J.L. (2007). Fetal microchimerism in women with breast cancer, Cancer Res. 67:9035-9038.
Publisher | Google Scholor - Gadi, V.K. Malone, K.E. Guthrie, K.A. Porter, P.L. Nelson J.L. (2007). Casecontrol study of fetal microchimerism and breast cancer, PLos One,3:1706.
Publisher | Google Scholor - Gadi, V.K. Malone, K.E. Guthrie, K.A. Porter, P.L.,Nelson J.L. (2008). Case-control study of fetal microchimerism and breast cancer. PLoS One, 3:1706.
Publisher | Google Scholor - Gadi, V.K. (2009). Fetal microchimerism and cancer. Cancer Lett. 276:8-13.
Publisher | Google Scholor - Galofré, J.C. (2012). Microchimerism in Graves' disease. J Thyroid Res. 724382.
Publisher | Google Scholor - Gilmore, G.L. Haq, B. Shadduck, R.K. Jasthy, S.L. Lister J. (2008). Fetal–maternal microchimerism in normal parous females and parous female cancer patients. Exp Hematol. 36:1073-1077
Publisher | Google Scholor - Holland E, foetus, J. Obstet. (1994). A case of transplacental metastasis of malignant melanoma from mother, Gynaecol Br Emp. 56:529-536.
Publisher | Google Scholor - Kamper-Jørgensen, M. Biggar, R.J. Tjønneland, A. Hjalgrim, H. Niels, (2012). Opposite effects of microchimerism on breast and colon cancer. Eur J Cancer. 48:2227-2235.
Publisher | Google Scholor - Khosrotehrani, K. Reyes, R.R. Johnson, K.L. Freeman, R.B. Salomon,et.al. (2007). Fetal cells participate over time in the response to specific types of murine maternal hepatic injury. Human Reproduction. 22:654-661.
Publisher | Google Scholor - Taştemir Korkmaz, D. Demirhan, O. Abat, D. Demirberk, B. Tunç, (2015). Microchimeric Cells, Sex Chromosome Aneuploidies and Cancer. Pathol Oncol Res. 21(4):1157-1165.
Publisher | Google Scholor - Jonsson, V. Tjonnfjord, G. Samuelsen, S.O. Johannesen, T. Olsen, et.al. (2007). Birth order pattern in the inheritance of chronic lymphocytic leukaemia and related lymphoproliferative disease, Leuk Lymphoma. 48:2387-2396.
Publisher | Google Scholor - Nguyen Huu, S. Oster, M. Avril, M.F. Boitier, F. Mortier, (2009). Fetal microchimeric cells participate in tumour angiogenesis in melanomas occurring during pregnancy. Am J Pathol. 174:630–637.
Publisher | Google Scholor - Nelson, J.L. (2009). Naturally acquired microchimerism: for better or for worse. Arthritis Rheum. 60(1):5-7.
Publisher | Google Scholor - O’Donoghue, K. Sultan, H.A. Al-Allaf, F.A. Anderson, J.R. Wyatt-Ashmead, et.al. (2008). Microchimeric fetal cells cluster at sites of tissue injury in lung decades after pregnancy. Reprod Biomed Online. 16:382-390.
Publisher | Google Scholor - Potter, J.F, Schoeneman, M. (1970). Metastasis of maternal cancer to the placenta and fetus, Cancer. 25:380–388.
Publisher | Google Scholor - Sawaya, H.H.B. Jimenez, S.A. Artlett, C.M. (2004). Quantification of fetal microchimeric cells in clinically affected and unaffected skin of patients with systemic sclerosis. Rheumatology. 43: 965-968.
Publisher | Google Scholor - Tan, X.W. Liao, H. Sun, L. Okabe, M. Xiao, (2005). Fetal microchimerism in the maternal mouse brain: a novel population of fetal progenitor or stem cells able to cross the blood– brain barrier? Stem Cells. 23(10):1443-1452.
Publisher | Google Scholor - Taştemir Korkmaz, D. Demirhan, O. Çetinel, N. Tunç, E. (2021). Fetal microchimerism and X chromosome aneuploidies in women with breast cancer. International Journal of Medical and Health Research. 7:31-38.
Publisher | Google Scholor - Wang, Y. Iwatani, H. Ito, H. Horimoto, N. Yamato, (2004). Fetal cells in mother rats contribute to the remodeling of liver and kidney after injury. Biochemical and Biophysical Research Communications. 325:961-967.
Publisher | Google Scholor