Research Article
The Efficacy of Antenatal Dexamethasone for Reducing Neonatal Complications in Late Preterm Infants: A Multicenter, Randomized Double-Blind Placebo-Controlled Trial
- Suppasiri Hayagangchart
- Sukanya Chaiyarah *
- Kiattisak Kongwattanakul
- Rattana Komwilaisak
Department of Obstetrics and Gynecology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand.
*Corresponding Author: Sukanya Chaiyarah, Department of Obstetrics and Gynecology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand.
Citation: Hayagangchart S, Chaiyarah S, Kongwattanakul K, Komwilaisak R. (2024). The Efficacy of Antenatal Dexamethasone for Reducing Neonatal Complications in Late Preterm Infants: A Multicenter, Randomized Double-Blind Placebo-Controlled Trial, Journal of Women Health Care and Gynecology, BioRes Scientia Publishers. 3(2):1-6. DOI: 10.59657/2993-0871.brs.24.027
Copyright: © 2024 Sukanya Chaiyarah, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: December 26, 2023 | Accepted: January 26, 2024 | Published: February 03, 2024
Abstract
Objective: To determine the effectiveness of giving dexamethasone before birth to reduce complications in late preterm infants.
Study design: randomized double-blind placebo-controlled trial.
Methods: 200 singleton pregnant women who were admitted due to preterm labour from April 2018 to April 2019 were randomized by block of four method into two groups. The study group (n=100) was prescribed dexamethasone 6mg intramuscularly every 12 hours for total 4 doses or until delivery and control group (n=100) who not receive dexamethasone.
Rate of neonatal respiratory distress, morbidity and mortality were assessed and analysed by Unpaired T-test, Mann Whitney U-test, and Chi-square test between both groups.
Results: there was no significant difference of primary outcome that is neonatal respiratory distress rate between dexamethasone group and control group (14.00% and 22.22%, RR (95%CI) =0.63(0.34-1.16, p 0.13). There was no difference of secondary outcomes; neonatal death, APGAR score <7 at 1 minute, APGAR score <7 at 5 minutes, need respiratory support, respiratory morbidities, hypoglycaemia, early neonatal jaundice, NEC, NICU admission, and length of hospital stay between both groups. Additional, maternal outcome (chorioamnionitis, postpartum endometritis, caesarean delivery) was no significant difference between two groups.
Conclusion: dexamethasone administration does not have a significantly reduced respiratory outcome even in late preterm.
Keywords: late preterm; respiratory distress; dexamethasone
Introduction
Premature birth remains a major problem in global health systems because it is a leading cause of new-born deaths, complications, and disabilities [1-4]. According to a recent report by the World Health Organization, the global average incidence of premature birth is about 9.6%, with higher rates in African and Asian countries [5]. In Thailand, the reported incidence of premature birth was 13.7% in 2010 at Siriraj Hospital [6] and 11.5% in 2013 at Srinagarindra Hospital [7].
The risk of death and complications in new-borns is higher in preterm infants with a gestational age of less than 34 weeks, and the risk increases with decreasing gestational age [8]. However, it has been found that 70% of preterm births are late preterm births [9-10]. Late preterm infants are defined as infants born between 34 and 36+6 weeks of gestation [11]. Although the risk of death and complications in this group is lower than in preterm infants born before 34 weeks of gestation, several studies have shown that late preterm infants still have higher rates of death and complications than term infants, such as respiratory complications, neonatal hypoxic-ischemic encephalopathy, intraventricular haemorrhage, hypoglycaemia, and the need for mechanical ventilation or admission to the neonatal intensive care unit (NICU) [4, 12-16]. This also increases the cost of care for these infants [16-18]. The main cause is often respiratory problems, such as respiratory distress syndrome, transient tachypnoea of the new-born (TTNB), and pneumonia [4, 19].
The use of corticosteroids in pregnant women with preterm labour to reduce the risk of complications and mortality in new-borns has strong evidence to support its use. Corticosteroids have been shown to reduce the risk of death, the frequency and severity of respiratory distress syndrome, and the incidence of intracranial haemorrhage and necrotizing enterocolitis in preterm infants born before 34 weeks of gestation [20-23]. As a result, the use of corticosteroids in pregnant women with preterm labour between 24 and 33+6 weeks of gestation is considered the standard of care from the past to the present [24, 25]. The most used types and doses of corticosteroids are betamethasone 12 mg intramuscularly twice, 24 hours apart, and dexamethasone 6 mg intramuscularly every 12 hours for a total of 4 doses [24]. Based on the available data, there is no evidence to support the superiority of one type of drug over another. Therefore, either type of drug can be chosen depending on the context of the healthcare facility and the choice of the treating physician.
Benefits of giving corticosteroids to pregnant women with late preterm labour, there have been increasing research studies, especially in the group of women given betamethasone. The initial research results showed that giving corticosteroids can reduce the incidence of respiratory distress syndrome in new-borns [26], but some studies found that giving the drug did not reduce respiratory complications in infants [27]. However, the study of Gyamfi-Bannerman C and colleagues in 2016, which is the largest population study with 2,827 participants, showed that giving betamethasone to pregnant women with late preterm labour can significantly reduce the rate of mechanical ventilation and severe respiratory complications in infants [28]. As a result, the American College of Obstetricians and Gynaecologists (ACOG) has recently recommended the use of betamethasone in pregnant women with late preterm labour [25]. However, there is still not enough data in the dexamethasone group.
In the context of Srinagar Indra Hospital, dexamethasone is given to all pregnant women with predicted to deliver at 24-33+6 weeks of gestation. However, for pregnant women with late preterm labour, there is no clear guidance because the lack of betamethasone and there is not enough evidence to support the benefits of using dexamethasone in this group. Therefore, the researchers need to study the effectiveness of giving dexamethasone before birth to reduce complications in late preterm infants. This will be beneficial for the care of pregnant women in the future.
Materials and Methods
Study population
The study was a multicentre, randomized double-blind placebo-controlled trial. The study sites are the delivery room of Srinagar Indra University Hospital, Faculty of Medicine, Khon Kaen and the delivery room of Khon Kaen regional Hospital. The study began after receiving approval from the Human Research Ethics Committee in April 2018 to April 2019. The inclusion criteria were women who are over 18 years old and are at high risk of delivering before 37 weeks of gestation (including women with preterm labour who have cervical dilation of at least 3 centimetres or cervical effacement of at least 80%, or who are scheduled to deliver preterm within 7 days of study enrolment due to medical indications such as severe preeclampsia, intrauterine growth restriction, preterm premature rupture of membranes, etc.). Exclusion criteria were women who have previously received corticosteroids during pregnancy, have severe infection or chorioamnionitis, have cervical dilation of 8 centimetres or more, have fetal abnormalities such as congenital malformations, non-reassuring fetal status requiring urgent delivery, fetal death in utero, or are allergic to corticosteroids.
Assessment of outcome
Participants were randomized by block of four method into two groups, the intervention group was prescribed dexamethasone 6 mg intramuscularly every 12 hours for totally 4 doses or until delivery and controlled group who received .9% NSS 1.5ml intramuscularly every 12 hours for totally 4 doses or until delivery.
The primary outcome was neonatal respiratory distress rate. Secondary outcome was neonatal death, APGAR score less than 7 at 1 minute, APGAR score less than 7 at 5 minutes, need respiratory support, respiratory morbidities, hypoglycaemia, early neonatal jaundice, NEC, NICU admission, length of hospital stay between both groups, and maternal outcome (chorioamnionitis, postpartum endometritis, caesarean delivery).
Statistical Analysis
Sample size was calculated to have type one error of 5 percent and 80 percent power to detect a reduction of 50 percent in rate of respiratory distress. Rate of respiratory distress in late preterm infant was assumed to be 28 percent based on Wang ML, et al [4]. Accordingly, the number of study population was at least 132 pregnant women in each group. Additional 10% loss the number of study population was at least 150 pregnant women in each group. All statistical analysis was done using SPSS program version 17.0. The patient’s baseline characteristics in each group were compared. Unpaired T-test for continuous quantitative variables with normal distribution. The Mann-Whitney U-test for discrete or nonnormal distribution quantitative variables and chi-square test for categorical variables, calculated risk-ratio as a measurement of relative risk, together with 95 percent confidence interval. P-value less than 0.05 was considered as significance.
Results
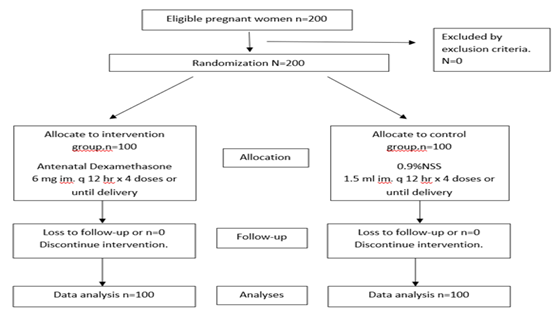
Two hundred pregnant women met the inclusion criteria and were randomized to dexamethasone group (n=100) or control group (n=100). Baseline characteristics of both groups; maternal age, parity, gestational age, and neonatal birth weight were no difference (Table 1).
Table 1: Baseline characteristics.
| Characteristics | Dexamethasone group (n=100) | Placebo group (n=100) | p-value |
| Maternal age (years), | |||
| Mean (± SD) | 27.45 ± 5.18 | 28.05 ± 5.82 | 0.4475 b |
| Parity | |||
| Nulliparous, N (%) | 38 (38.38) | 46 (46) | 0.277 a |
| Multiparous, N (%) | 61 (61.62) | 54 (54) | |
| Gestational age, N (%) | |||
| < 34> | 11 (15.28) | 12 (16) | 0.888 a |
| 35-35+6 weeks | 17 (23.61) | 20 (26.67) | |
| 36-36+6 weeks | 44 (61.11) | 43 (57.33) | |
| Neonatal birth weight (grams) | |||
| Mean (± SD) | 2688.13 ± 412.91 | 2705.92 ± 440.01 | 0.7714 b |
*a chi square test; *b independent t-test
Figure 1: Enrollment, randomization, treatment, and follow-up of patients.
There was no significant difference of primary outcome that is neonatal respiratory distress rate between dexamethasone group and control group (14.00% and 22.22%, RR (95% CI) = 0.63(0.34-1.16, p 0.13) (Table 2). Furthermore, there was no difference of secondary outcomes; neonatal death, APGAR score < 7>
Table 2: Neonatal outcome of late preterm infants.
| Characteristics | Dexamethasone (n=100) | Placebo (n=100) | p-value | Relative risk (95% CI) |
| Primary outcome, N (%) | ||||
| Respiratory distress | 14 (14) | 22 (22.22) | 0.137 | 0.63 (0.34 - 1.16) |
| Secondary outcomes, N (%) | ||||
| Neonatal death | 0 | 0 | - | - |
| APGAR score < 7> | 5 (5) | 8 (8) | 0.395 | 0.63 (0.21 - 1.84) |
| APGAR score < 7> | 0 | 1 (1) | - | - |
| Need respiratory support | 13 (16.5) | 20 (20.00) | 0.187 | 0.65 (0.34 - 1.23) |
| Respiratory morbidities | 8 (8.42) | 17 (17.71) | 0.066 | 0.48 (0.22 - 1.05) |
| Transient tachypnea of the newborn | 1 (1.14) | 4 (4.82) | 0.192 | 0.24 (0.03 - 2.07) |
| Pneumonia | 7 (7.45) | 13 (14.13) | 0.15 | 0.53 (0.22 - 1.26) |
| Morbidities in others system | 31 (31) | 34 (34.34) | 0.615 | 0.9 (0.61 - 1.35) |
| Hypoglycemia | 1 (1.43) | 0 | - | - |
| Early neonatal jaundice | 18 (20.69) | 14 (17.72) | 0.629 | 1.17 (0.62 - 2.19) |
| NEC | 0 | 0 | - | - |
| NICU admission | 16 (16) | 24 (24.24) | 0.152 | 0.66 (0.37 - 1.17) |
| Length of hospital stay (day) | ||||
| Mean ± SD | 3.95 ± 3.57 | 4.49 ± 4.24 | - | - |
| Medin (p25 – p75) | 3 (2 – 4) | 3 (2 – 4) | 0.4387* | - |
Table 3: Maternal Outcomes.
| Characteristics | Dexamethasone (n=100) | Placebo (n=100) |
| Chorioamnionitis, N (%) | 0 | 0 |
| Postpartum endometritis, N (%) | 0 | 0 |
| Cesarean delivery, N (%) | 13 (13) | 22 (22.22) |
Discussion
70% of preterm births are late preterm births [9-10]. The initial research results showed that giving corticosteroids can reduce the incidence of respiratory distress syndrome in new-borns [26], but some studies found that giving the drug did not reduce respiratory complications in infants [27].
Our study shows that administration of dexamethasone in gestational age 34 to 36 weeks 6 days women with preterm labor not significantly reduce respiratory complications in infants also neonatal death, APGAR score less than 7 at 1 minute, APGAR score less than 7 at 1 minute, APGAR score less than 7 at 5 minutes, need respiratory support, respiratory morbidities, hypoglycaemia, early neonatal jaundice, NEC, NICU admission, and length of hospital stay between both groups that was difference from the study of Farid A. Kassab [29] and Attawatanakul A [30]. However, the sample size of our study was less than calculated sample size that we need 300 participants, but we included only 200 participants. We could not conclude whether the number of dexamethasone injections will have different effects on respiratory outcome because of limitation of number of participants. Nevertheless, administration of dexamethasone in gestational age 34 to 36 weeks 6 days women with preterm labour trend to reduce RDS (dexamethasone 14% vs control 22.22 %), APGAR score less than 7 at 1 minute (dexamethasone 5%vs control 8%), Need respiratory support (dexamethasone 16.50%vs control 20.00%), respiratory morbidities (dexamethasone 8.42%vs control 17.71%), and NICU admission (dexamethasone 16.00%vs control 24.24%). We found that only one case had hypoglycaemia in new-borns. Furthermore, we found that early neonatal jaundice in the control group was less than dexamethasone group.
The strength of the present study was 1) we assessed the effects of dexamethasone on the basis of standard practice 2). there was no participant that loss to follow up in our study.
This study concluded that dexamethasone does not have a significantly reduced respiratory outcome even in late preterm. A further study should be conducted by increased number of participants to have enough power to detect the rate of RDS.
Conclusion
Our study showed that dexamethasone does not have a significantly reduced respiratory outcome even in late preterm.
Declarations
Conflict of interest
The authors declare that there was no conflict of interest.
Acknowledgement
We would like to thank the women who participated the trial, residency training, nurse or staff at each site that well co-operate in this trial. We also thank the department of Obstetrics and Gynecology, Khon Kaen University for providing resources of the research.
References
- Kinney MV, Lawn JE, Howson CP, Belizan J. (2012). 15 million preterm births annually: what has changed this year? Repord Health, 9:28.
Publisher | Google Scholor - Tucker JM, Goldenberg RL, Davis RO, Copper RL, Winkler CL, Hauth JC. (1991). Etiologies of preterm birth in an indigent population: is pre¬vention a logical expectation? Obstet Gynecol, 77(3):343-347.
Publisher | Google Scholor - Huddy CL, Johnson A, Hope PL. (2001). Educational and behavioral problems in babies of 32–35 weeks gestation. Arch Dis Child Fetal Neonatal Ed, 85:23-28.
Publisher | Google Scholor - Wang ML, Dorer DJ, Fleming MP, Catlin EA. (2004). Clinical outcomes of near-term infants. Pediatrics, 114:372-376.
Publisher | Google Scholor - Stacy Beck, Daniel Wojdyla, et al. (2010). The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bulletin of the World Health Organization, 88:31-38
Publisher | Google Scholor - Chawanpaiboon S, Kanokpongsakdi S. (2011). Preterm birth at Siriraj Hospital: a 9-year period review (2002–2010). Siriraj Med J, 63(5):143-146.
Publisher | Google Scholor - Kiatsuda D, Thinkhamrop J, Prasertcharoensuk W. (2016). Success rate in preterm uterine contraction inhibition with tocolytic agents in tertiary care center. International Journal of Women’s Health, 8:663-667.
Publisher | Google Scholor - World Health Organization (WHO): Recommendations on interventions to improve preterm birth outcomes2015.
Publisher | Google Scholor - Martin JA, Hamilton BE, Ventura SJ, et al. (2012). Births: Final data for 2010. Natl Vital Stat Rep, 61:1.
Publisher | Google Scholor - Imkesorn R, Hluangdansakul W, et al. (2015). Comparison of Respiratory Distress Syndrome (RDS) between Gestational Age 34 Weeks and 35-36 weeks. Thai Journal of Obstetrics and Gynecology, 13:18-24.
Publisher | Google Scholor - Raju TN, Higgins RD, Stark AR, Leveno KJ. (2006). Optimizing care and outcome for late preterm (near term) infants: a summary of the workshop sponsored by the National Institute of Child Health and Human Development. Pediatrics, 118:1207.
Publisher | Google Scholor - Shapiro-Mendoza CK, Tomashek KM, et al. (2008). Effect of late-preterm birth and maternal medical conditions on newborn morbidity risk. Pediatrics, 121(2):223.
Publisher | Google Scholor - Engle WA, Tomashek KM, Wallman C. (2007). Committee on Fetus and Newborn, American Academy of Pediatrics Late-preterm
Publisher | Google Scholor - Leone A, Ersfeld P, et al. (2012). Neonatal morbidity in singleton late preterm infants compared with full-term infants. Acta Paediatr, 101(1):6.
Publisher | Google Scholor - Sonia Marrocchella, Veronica Sestilli, Ugo Indraccolo, et al. (2014). Late preterm births: A retrospective analysis of the morbidity risk stratified for gestational age. Spinger Plus, 3:114.
Publisher | Google Scholor - Ryan W. Loftin, Mounira Habli, Candice C. Snyder, et al. (2010). Late preterm birth. Reviews in Obstetrics et Gynecology, 3(1):10-19.
Publisher | Google Scholor - McLaurin KK, Hall CB, Jackson EA, et al. (2009). Persistence of morbidity and cost differences between late-preterm and term infants during the first year of life. Pediatrics, 120(6):1390.
Publisher | Google Scholor - Bird TM, Bronstein JM, Hall RW, et al. (2010). Late preterm infants: birth outcomes and health care utilization in the first year. Pediatrics, 126:311.
Publisher | Google Scholor - Consortium on safe labor, Hibbard JU, Wilkins I, et al. (2010). Respiratory morbidity in late preterm births. JAMA, 304:419.
Publisher | Google Scholor - Roberts D, DalzielS. (2010). Antenatal corticosteroids for accelerating fetal lung maturation for woman at risk of preterm birth. Cochrane Database Syst Rev, 9:1173.
Publisher | Google Scholor - ACOG committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin no.127: Management of preterm labor. Obstet Gynecol, 119:1308-1317.
Publisher | Google Scholor - (2000). Antenatal corticosteroids revisited: repeat courses. NIH Consensus Statement, 17:1-18.
Publisher | Google Scholor - Liggins GC, Howie RN. (1972). A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics, 50(6):515-525.
Publisher | Google Scholor - (2016). Management of preterm labor. Practice Bulletins No.171: American college of Obstetricians and Gynecologists, Obstet Gynecol, 128:155-164.
Publisher | Google Scholor - (2017). Antenatal Corticosteroid Therapy for Fetal Maturation. ACOG Committee Opinion No.713: American college of Obstetricians and Gynecologists. Obstet Gynecol, 130:102-109.
Publisher | Google Scholor - Balci O, Ozdemir S, Mahmoud AS, et al. (2010). The effect of antenatal steroids on fetal lung maturation between the 34th and 36th week of pregnancy. Gynecol Obstet Invest, 70:95-99.
Publisher | Google Scholor - Porto AM, Coutinho IC, et al. (2011). Effectiveness of antenatal glucocorticoid in reducing respiratory disorders in late preterm infants: randomized clinical trial. BMJ, 342:1696.
Publisher | Google Scholor - Gyamfi-Bannerman C, Thom EA, Blackwell SC, Tita AT, Reddy UM, Saade GR, et al. (2016). Antenatal betamethasone for women at risk for late preterm delivery. NICHD Maternal-Fetal Medicine Units Network. N Engl J Med, 374:1311-1320.
Publisher | Google Scholor - Farid A. Kassab. (2013). The effect of antenatal steroid on fetal outcome after the 34 weeks of pregnancy in women at high risk of preterm labor. AAMJ, 4:262-272.
Publisher | Google Scholor - Attawattanakul N, Tansupswatdikul P. (2015). Effects of antenatal dexamethasone on respiratory distress in late preterm infant: A randomized controlled trial. Thai Journal of Obstetrics and Gynecology, 23:25-33
Publisher | Google Scholor - Ngamjarus C, Chongsuvivatwong V. (2014). n4Studies: Sample size and power calculations for iOS. The Royal Golden Jubilee Ph.D. Program - The Thailand Research Fund&Prince of Songkla University.
Publisher | Google Scholor - (2015). The management of preterm labour and preterm rupture of membranes. RTCOG clinical practice guideline, 15-41.
Publisher | Google Scholor