Review Article
The Concepts of Angiogenesis and its Implications in Oncology
Professor Radiation Oncology, J.K Cancer Institute, Kanpur, India.
*Corresponding Author: M.Q BAIG, Professor Radiation Oncology, J.K Cancer Institute, Kanpur, India.
Citation: M.Q Baig. (2023). The Concepts of Angiogenesis and its Implications in Oncology. International Journal of Cancer Management and Research, BRS Publishers. 1(1):1-5. DOI: 10.59657/2996-4563.brs.23.001
Copyright: © 2023 M.Q BAIG, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: December 15, 2022 | Accepted: January 20, 2023 | Published: January 24, 2023
Abstract
Angiogenesis is the growth of new blood vessels and is a key process which occurs during both physiological and pathological disease processes. Knowledge of the mechanisms through which this process is initiated and maintained will have a significant impact on the many of the solid tumors in field of medical oncology. Pathological angiogenesis occurs in major diseases such as cancer, diabetic retinopathies, age-related macular degeneration and atherosclerosis.
Keywords: angiogenesis; medical oncology; solid tumors
Introduction
Approval of several anti-angiogenesis drugs for systemic therapy for several malignances have been developed, like bevacizumab a humanized monoclonal antibody to vascular endothelial growth factor VEGF, however the success of bevacizumab in combination with chemotherapy in metastatic breast cancer and metastatic colorectal cancer is well established and as monotherapy of bevacizumab in case of metastatic renal cancer approved till date. Era of antiangiogenic drugs began with the landmark article published in 1971 in New England Journal of Medicine by Folkman, the content of above publication was given below,
- Solid tumors will not grow beyond 2mm in size unless they induce and sustain new blood supply to provide oxygen and nutrients for their growth of tumor mass.
- Up to 2mm size tumor may derive its oxygen and nutrients by passive diffusion.
- When tumor cell population begins to produce TAF tumor angiogenic factors which creates revascularizations by endothelial cells these endothelial cells of nearby pre-existing blood vessels began to migrate, divide and formed tubular structures to form new blood vessels these blood vessels infiltrate in tumor mass to established new blood supply for the progress of tumor growth.
- Administration of anti-angiogenesis agents for example Anti TAF tumor angiogenesis factor specific neutralizing antibodies will block tumor angiogenesis process, but such tumor shrinkage would never be completely because microscopic tumor which size 2mm or less can survive without new vessels so this type of therapy never be called curative Dr Folkman further described in his reports following- He described nature of angiogenic switch present in tumors, first stimulator of angiogenesis, are Basis fibroblast growth factor and discovery of several endogenous anti-angiogenesis inhibitors, after so many twists and turns, the concepts of therapeutic role of angiogenesis has been finally formulated,
- Certain angiogenic drugs seems to have no clinical benefits when used as monotherapy but does have good role when it is combined with chemotherapy drugs.
- In addition to stimulators of angiogenisis process there are number of endogenous antiangiognisis factors down regulatory effect on them can lead to progress of tumors.
- Angiogenesis can also contribute in growth of liquid tumors like hematological malignancies not just for only solid tumors due to expression of number of growth factors like cytokines, cytokinines by activated endothelial cells at newly formed blood vessels sites, as well as bone marrow which promote survival and growth of tumor cell populations.
Discussion
A-Process of formation of new blood vessels:
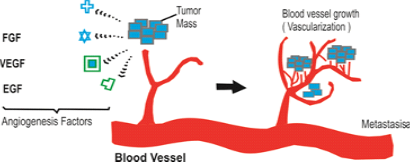
Figure 1: Steps of Angiogenesis and Metastasis
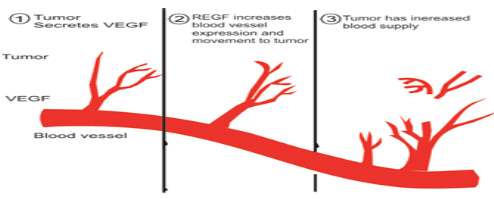
There are number of sequential events involved in the development of new capillary blood vessels in ongoing tumor mass. The first step in the formation of new blood vessels is breaking of basement membrane of parental venule (pre-existing small blood vessels) that create moment of endothelial cells towards adjacent tumor cells this process stimulated by proangiogenic factors secreted by tumor cell mass, these proangogenic factors Known as Matrix etalloproteinase and urokinase plasminogen activator. (They arebasically proteolysis enzymes) This philosophy of above two enzymes (proangiogenic factors) leads to research to form drugs which inhibit such enzyme. Second step in formation of new blood vessels is migration of endothelial cells as well as division of these mature endothelial cells adjacent to tumor moss and finally formation of new basement membrane of juvenile blood vessels these new angiogenic blood vessels of tumor cells established the further growth of tumor cells populations, apart from this some endothelial proginator cells formed in bone marrow from here these goes to main blood vessels supply via this these proginators reaches to tumor cell mass to stimulate division of mature endothelial cells in order to form new blood vessels.
B-Pericytes
Pericytes is a single layer of periendothlial smooth muscle cells which modulate endothelial cell functions and critical for the development of mature vascular network since role of pericytes in helping survival of endothelial cells thus pericytes emerged as an important therapeutic target for antiangiogenic therapy.
C-Molecular Mediators of Tumor Angiogenisis
Several angiogenic growth factors found which modulates tumor angiogenesis some working directly some working indirectly thus divided as,
- Directly acting factors
- Indirectly acting growth factors.
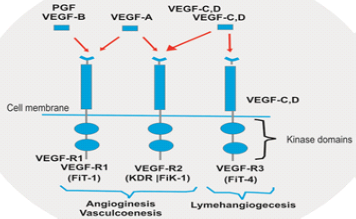
Directly acting growth factors include VEGF family include Tyrosin kinase, angiopoetine, notch singling receptor specially notch 4, NOTCH 4? is a member of notch family being basically a Trans Membrane RECEPTOR express primarily on endothelial cells of blood vessels? All factors have high degree of specificity for endothelial cells associated neovascularization. VEGF another name is VPF vascular Permeability factor, VEGF produced by primary tumor cells, macrophages, platelets cells, VEGF also plays role in normal physiological functions such as bone marrow formation, Haemopoiesis, wound healing not only limited to solid tumors but also hematological malignancies leukemia’s and lymphomas. Types of VEGF -VEGF 1, VEGF 2, VEGF3 OUT OF THAT VEGF 1 AND 2 regulate angiogenesis and VEGF regulate lymph angiogenesis. VEGF is protein with vascular permeability that is secreted by tumor cells.
Indirectly acting angiogenic factors those who amplify angiogenic process are TNF a and TNF b, transforming growth factors, Inflammatory cytokinines such as IL6 and IL8, Granulocyte stimulating factor, PDGF platelet derived growth factor, estrogen and androgen hormone as well. VGEF was discovered in 1989 and replaced to be highly specific and potent mitogen for vascular endothelial cells.
Figure 2: Factors Which Promotes Angogenisis
There are four roles of VEGF by which it promotes tumor angiogenisis it can stimulate endothelial cells division and induce endothelial cells migrations also enhanced endothelial cells survival and mobilizes endothelial progenitors’ cells from bonemarrow to the site of tumorogenesis, elevated expression of VGEF commonly associated with tumor hypoxia so hypoxia in tumor cells leads to over expression of VGEF thus leads to neovascularization of tumor cell mass.
Figure3: how new blood vessels develop for tumor progression
D-Endogenus Inhibitors of Tumor Angiogenisis
In addition to multiple factors stimulating the angiogenesis there are inhibits molecules of angiogenesis process as well that inhibitor is a large glycoprotein molecule present in Extracellular matrix named Trombospondin-1 (TSP-1) P53 gene was found to regulate TPS-1 level, loss of P53 gene associated with down regulation of TSP-1 expression. Second inhibitor is VASOINHIBIN a protein which is secreted by Endothelial cells a stimulation of angiogenic factors VEGF, the Biology of Endogenous angiogenisis inhibitors is that they maybe induce by other cancer therapy to have added effect for example some antiangiogenic treatment strategy such as low dose metronomic chemotheraphy or doxiycline are known to induce elevated levels of TSP-1.
E-Strategies for Development of Antiangiogenic Drugs
Most obvious strategy would be development of drugs that neutralize proangiogenic growth factors such as VEGF. All approved antiangiogenic drugs either block VEGF or VEGF tyrosin kinase receptor example Bevacizumab drug developed to neutralize VEGF in addition to antibody/protein therapy a large number of small molecule and receptor tyrosin kinase inhibitors have been developed to block VEGF receptor phosphorylation such drugs are SUNITINIB and SORAFENIB these drugs action is not only inhibition of angiogenesis but also they directly act by inhibition of tumor cell. Bevacizumab when bevacizumab treatment would be more helpful, use alone not effective but when use along chemotherapy drugs they prove to have good results, other mode of treatment and drugs those having antiangiogenic effect or example radiation treatment, Cetuximab or Transtuzumab (targeting Her-2 antibody) do have down regulatory effect on VEGF and up regulatory effect on TSP-1.
How antioangiogenic drugs enhanced the effects of radiation and chemotherapy? Antioangiogenic drugs actually increase tumor oxygenation and blood supply thus increase intratumor level of chemotherapy as well as increase oxygen level also increases the effects of radiotherapy.
F-Markers of Tumor Angiogenisis
Possible markers of tumor angiogenesis are circulatory blood proteins VEGF, Proangiogenic growth factors, circulatory endothelial progenitors’ cells. Biomarkers are elevated levels of VEGF are associated with poor prognosis, thus if high levels of VEGF, bevacizumab treatment would be more helpful, VEGF receptors diagnosis and test require tissue specimen for immunohistochemistary study but the tissue specimen must be from:
Benefits of Anti-Vegf Therapy
- Inhibit the new vessels growth in tumor tissue.
- Blockade OF development of endothelial progenitor cells
- Normalization of vasculature thus decrease permeability of and increase chemotherapy drug delivery to tumor tissue.
- Direct effect on tumor as antitumor effect.
- Inhibition of damage of endothelial cells by chemotherapy drugs.
- Immunomodulatory effect. For the tumors other than renal cell carcinoma anti VEGF therapy only provide benefits when it given in combination with chemotherapy drugs, Tyrosin kinase inhibitors in metastatic colorectal cancer enhanced its effect when given with chemotherapy drugs like metastatic breast cancer bevacizumab augments the effects of paclitaxil based chemotherapy.
Toxicity of anti vegf therapy:
- Hypertension,
- Proptienurea,
- Bowel perforation,
- Anti-thrombotic events Hypertensions do occur due to anti VEGF causes inhibition of endothelial cells derived nitric oxide which is known vasodilator.
Why cause protein urea because it causes damage to vascular endothelial cells of glomeruli which leads to protein urea Gastro intestinal perforation do occur in 1.5 to 5.5 % patients receiving bevasuzimab, because it causes GI ulceration which further leads to gastrointestinal perforations Bevasuzimab induces VEGF inhibition which results in cholesterol emboli syndrome CES (HTN, GI PERFORATION, ESIONOPHELIA) which leads to mesenteric vessels ischemia which further leads to perforation.
Conclusion
Basis of angiogenesis within the tumor ecosystem cellular and molecular components is provided. Clinical evidence has demonstrated the effectiveness of traditional vessel-directed antiangiogenics, stressing on the important role of angiogenesis in tumor establishment, dissemination, and growth. Particular focus is placed on the interaction between tumor cells and their surrounding ecosystem, which is now regarded as a promising target for the development of new antiangiogenics drugs which having proving benefits in colorectal cancer, breast cancer as well as in lung cancer (NSCLC) Non-small cell lung carcinomas.
References
- Lacas B, Carmel A, Landais C, Wong SJ, Licitra L, Tobias JS et al. (2021). MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 107 randomized trials and 19,805 patients, on behalf of MACH-NC Group. Radiother Oncol. 156:281-293.
Publisher | Google Scholor - Juarez JE, Choi J, St John M, Abemayor E, TenNapel M and Chen MA. (2017). Patterns of care for elderly patients with locally advanced head and neck cancer. Int J Radiation Oncol Biol Phys. 98(4):767-774.
Publisher | Google Scholor - Murthy VH, Krumholz HM, Gross CP. (2004). Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 291:2720-2726
Publisher | Google Scholor - Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C et al. (2011). Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 12:127-136.
Publisher | Google Scholor - Goyal G, Patil VM, Noronha V, Joshi A, Khaddar S, Kakkar S et al. (2018). Once-a-week versus once-every-3-weeks cisplatin in patients receiving chemoradiation for locally advanced head-and-neck cancer: A survey of practice in India. Cancer Res Stat Treat. 1:63-67.
Publisher | Google Scholor - American Joint Committee on Cancer. AJCC cancer staging handbook, 7th edn. Springer: New York; 2010.
Publisher | Google Scholor - Cox JD, Stetz J and Pajak TF. (1995). Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int. J. Radiation Oncology Biol. Phys. 31:1341-1346.
Publisher | Google Scholor - Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al. (2009). New Response Evaluation Criteria in Solid Tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 45:228-247.
Publisher | Google Scholor - VanderWalde NA, Meyer AM, Deal AM, Layton JB, Liu H, Carpenter WR et al. (2014). Effectiveness of Chemoradiation for head and neck cancer in an older patient population. Int J Radiation Oncol Biol Phys. 89(1):30-37.
Publisher | Google Scholor - Nyugen NP, Vock J, Chi A, Vinh-Hung V, Dutta S, Ewell L et al. (2012). Impact of intensity-modulated and image-guided radiotherapy on elderly patients undergoing chemoradiation for locally advanced head and neck cancer. Strahlenther Onkol. 188:677-685.
Publisher | Google Scholor - Amini A, Jones BL, McDermott JD, Serracino HS, Jimeno A, Raben D et al. (2016). Survival outcomes with concurrent chemoradiation for elderly patients with locally advanced head and neck cancer according to the National Cancer Data Base. Cancer. 122(10):1533-1543.
Publisher | Google Scholor - Nguyen-Tan PF, Zhang Q, Ang KK, Weber RS, Rosenthal DI, Soulieres D et al. Randomized Phase III Trial to Test Accelerated Versus Standard Fractionation in Combination with Concurrent Cisplatin for Head and Neck Carcinomas in the Radiation Therapy Oncology Group 0129 Trial: Long-Term Report of Efficacy and Toxicity. J ClinOncol 32:3858-3867
Publisher | Google Scholor - Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS et al. Randomized Phase III Trial of Concurrent Accelerated Radiation Plus Cisplatin with or Without Cetuximab for Stage III to IV Head and Neck Carcinoma: RTOG 0522. J ClinOncol 32:2940-2950.
Publisher | Google Scholor - Gebre-Medhin M, Brun E, Engstrom P, Cange HH, Hammarstedt-Nordenvall L, Reizenstein J et al. (2020). ARTSCAN III: A Randomized Phase III Study Comparing Chemoradiotherapy with Cisplatin Versus Cetuximab in Patients with Locoregionally Advanced Head and Neck Squamous Cell Cancer. J ClinOncol. 39:38-47.
Publisher | Google Scholor - Viani GA, Faustino AC, Danelichen AFB, Matsuura FK, Neves LVF, Fernandes MH et al. (2021). Radiotherapy for locally advanced head and neck cancer in elderly patients: results and prognostic factors a single cohort. Rep Pract Oncol Radiother. 26(1):12-19.
Publisher | Google Scholor - Haehl E, Rühle A, David H, Kalckreuth T, Sprave T, Stoian R et al.et al. (2020). Radiotherapy for geriatric head-and-neck cancer patients: what is the value of standard treatment in the elderly? Radiat Oncol. 15: 31.
Publisher | Google Scholor - Müller von der Grün J, Martin D, Stöver T, Ghanaati S, Rödel C, Balermpas P. (2018). Chemoradiotherapy as Definitive Treatment for Elderly Patients with Head and Neck Cancer. Biomed Res Int.
Publisher | Google Scholor - Kunkler IH, Audisio R, Belkacemi Y, Betz M, Gore E, Hoffe S et al. (2014). SIOG Radiotherapy Task Force. Review of current best practice and priorities for research in radiation oncology for elderly patients with cancer: the International Society of Geriatric Oncology (SIOG) task force. Ann Oncol. 25(11):2134-2146.
Publisher | Google Scholor - Ward MC, Reddy CA, Adelstein DJ, Koyfman SA. (2016). Use of systemic therapy with definitive radiotherapy for elderly patients with head and neck cancer. A National Cancer Data Base analysis. Cancer. 122:3472-3483.
Publisher | Google Scholor - Szturz P, Wouters K, Kiyota N, Tahara M, Prabhash K, Noronha V et al. (2017). Weekly Low-Dose Versus Three-Weekly High-Dose Cisplatin for Concurrent Chemoradiation in Locoregionally Advanced Non-Nasopharyngeal Head and Neck Cancer: A Systematic Review and Meta-Analysis of Aggregate Data. Oncologist. 22(9):1056-1066.
Publisher | Google Scholor - The World Bank. (2019) Life expectancy at birth, total (years) – India.
Publisher | Google Scholor - Sommers LW, Steenbakkers RJHM, Bijl HP, Vemer-van den Hoek JGM, Roodenburg JLN, Oosting SF et al. (2017). Survival patterns in elderly head and neck squamous cell carcinoma patients treated with definitive radiation therapy. Int J Radiot Oncol Biol Phys. 98(4):793-801.
Publisher | Google Scholor - Kenis C, Decoster L, Puyvelde KV, De Grève J, Conings G, Milisen K et al. (2014). Performance of Two Geriatric Screening Tools in Older Patients with Cancer. Journal of Clinical Oncology. 32(1):19-26.
Publisher | Google Scholor