Research Article
The Activity of the Proapoptotic Protein Caspase-3 Changes in Glial Cells of the Peritumoral Zone of Secondary Brain Tumors, and its Relationship with Intraoperative Photodynamic Therapy
- Darya Sitovskaya 1,2*
- Darya Makshakova 2
- Tatyana Sokolova 1
- Konstantin Kukanov 1
- Anastasiia Nechaeva 1
- Yulia Zabrodskaya 1
1Polenov Neurosurgical Institute, Branch of Almazov National Medical Research Centre, St. Petersburg, Russia.
2 Department of Pathology with a course of forensic medicine named after D.D. Lochov, St. Petersburg State Pediatric Medical University, Saint-Petersburg, Russia.
*Corresponding Author: Darya Sitovskaya, Polenov Neurosurgical Institute, Branch of Almazov National Medical Research Centre, St. Petersburg, Russia.
Citation: Sitovskaya D, Makshakova D, Sokolova T, Kukanov K, Nechaeva A, et.al. (2024). The Activity of the Proapoptotic Protein Caspase-3 Changes in Glial Cells of the Peritumoral Zone of Secondary Brain Tumors, and its Relationship with Intraoperative Photodynamic Therapy, Clinical Research and Reports, BioRes Scientia Publishers. 2(6):1-10. DOI: 10.59657/2995-6064.brs.24.034
Copyright: © 2024 Darya Sitovskaya, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: July 16, 2024 | Accepted: August 03, 2024 | Published: August 09, 2024
Abstract
The metastasis of solid tumors to the central nervous system is a pressing issue due to its association with poor prognosis and low survival rates. Recent studies have shown that glial cells can promote metastasis even before the appearance of tumor cells, highlighting the need for new treatment methods. One potential method is intraoperative photodynamic therapy (PDT), which has been shown to improve the success of neuro-oncological surgeries and local control. However, the role of glial cell apoptosis in the development of brain metastases and the impact of PDT on apoptosis are still unclear. In this study, biopsy samples were collected from 25 patients undergoing surgery at the Polenov Neurosurgical Institute, including 16 women and 9 men with an average age of 63.04 years (ranging from 39 to 78 years). PDT was administered using the drug "Photoditazine" at a dosage of 1 mg per 1 kg of body weight. Histological sections stained with hematoxylin and eosin, as well as immunohistochemical (IHC) reactions with a rabbit polyclonal antibody to active caspase-3, were analyzed in the pathology department. The results showed that in the peritumoral zone of metastases, oligodendrocytes were the main cells undergoing apoptotic death, and PDT also induced apoptosis in astrocytes. The use of PDT significantly increased apoptosis in brain tissue (p<0.05). These findings suggest that targeting gliocyte apoptosis and caspase-dependent apoptosis mechanisms may be a potential therapeutic approach in the comprehensive treatment of metastatic carcinomas.
Keywords: caspase-3; apoptosis; peritumoral zone; brain metastasis; photodynamic therapy
Introduction
Metastasis of solid tumors to the central nervous system is a significant issue, as brain metastases are linked to a poor prognosis, regardless of their origin [1]. Despite specific antitumor therapy, the one-year survival rate remains low at only 20% [1-3]. Understanding the mechanisms of metastasis in the central nervous system is crucial for developing effective treatment approaches for patients with this condition, ultimately leading to improved prognosis. One promising treatment method in oncology is intraoperative photodynamic therapy (PDT) [4-5]. In Russia, several studies have demonstrated the clinical effectiveness of PDT in patients with glial tumors and secondary metastatic tumors. However, this technique is not yet included in clinical recommendations for providing high-tech neurosurgical care [6-7].
A certain "organotropism" has been described for several primary malignant solid tumors. This phenomenon may be partly explained by the anatomical proximity between organs. For example, the liver collects venous drainage from the colon system through the portal vein, making it a common site for metastasis of colon carcinoma [8]. However, in the past two decades, research has provided compelling evidence for the existence of cellular and molecular programs that direct tumor cells to specific organs [1]. The premetastatic niche concept, in particular, postulates that soluble factors and extracellular vesicles produced by cells at primary tumor sites can alter the microenvironment of distant organs in various ways to accommodate traveling cancer cells and facilitate their growth [9-10]. In 1889, English surgeon Stephen Paget formulated his landmark “seed and soil” theory, in which metastatic cancer cells are the “seeds” and the host microenvironment of the body is the “soil”. This theory has determined the direction of research into organ-specific metastases [11]. However, it is important to note that in some patients, metastases are found in the brain but not in the liver, for example [12-13]. This suggests that tumor cells may use specialized mechanisms to penetrate the highly selective blood-brain barrier, which is difficult for cancer cells to penetrate due to its tightly packed endothelial cells [14].
But it is not only the availability of the organ that determines where the metastatic "seed" will settle. The brain microenvironment also plays a crucial role, as suggested by the "seed and soil" hypothesis. Under certain conditions, certain cell populations may support the metastasis of cancer cells and promote their growth and proliferation [1]. For example, glial cells have been shown to have prometastatic effects, even before the appearance of tumor cells. Other glial cells can create a microenvironment that is conducive to the growth and proliferation of newly extravasated cancer cells or their clusters. In a model of metastatic melanoma, activated glial fibrillary acid protein (GFAP)-positive astrocytes produced pro-inflammatory molecules such as CCL2, IL-1β, and IL-6, which enhanced tumor growth [15]. Additionally, activated astrocytes, whose primary function is to protect neurons from toxic substances, can also be utilized by tumor cells to protect against chemotherapy drugs, suggesting potential new approaches to treating this fatal disease [16].
Neuroinflammation, which is associated with the development of apoptosis in glial cells and neurons, has been linked to the progression of many serious neurological diseases, including drug resistance and epilepsy [17-18]. However, the role of glial cell apoptosis in the development of brain metastases, as well as the effect of intraoperative photodynamic therapy on apoptosis, remains unclear.
The purpose of this study is to investigate the activity of the proapoptotic protein caspase-3 in the peritumoral zone of secondary brain tumors and to evaluate any changes in its activity under the influence of intraoperative photodynamic therapy.
Material and methods
Study Design and Patients
The study utilized a case-control design and involved obtaining biopsy material from tumor fragments and brain tissue from the perifocal zone of 25 patients at Polenov Neurosurgical Institute. Of these patients, 16 were women and 9 were men, with ages ranging from 39 to 78 years and an average age of 63.04±10.6 years. The patients underwent microsurgical interventions with metabolic navigation, and 9 of them also underwent PDT using the technique described below. Tissue samples were collected using a standard method, including fragments from the tumor zone, the cortex above the tumor, the perifocal zone, the perifocal zone 1 cm from the tumor bed, and the perifocal zone after PDT. Each fragment was assigned a digital code and label. The comparison group for histological examination and IHC consisted of 15 adult patients (10 men and 5 women) with an average age of 45.1±10 years who had died from somatic diseases such as acute myocardial infarction, gastric ulcer with bleeding, mesenteric thrombosis, or pulmonary embolism within 6 hours prior to autopsy. These patients had no history of neurological disorders.
Intraoperative photodynamic therapy
Intraoperative photodynamic therapy was performed on 9 patients using the drug "Photoditazine" from the limited liability company (LLC) "Veta-Grand" (Russia). The drug was administered intravenously 1.5-2 hours before the planned tumor removal, diluted in 200 ml of physiological solution at a rate of 1 mg per 1 kg of patient body weight. A Latus 2.5 semiconductor laser from Semiconductor Devices LLC (St. Petersburg, Russia), with a wavelength of 665 nm, was used for the therapy. This wavelength corresponds to the absorption peak of photoditazine. The treatment was carried out with the assistance of fluorescent control, targeting only areas of tumor tissue that were inaccessible for microsurgical removal. The unique aspect of this technique was that the duration of irradiation was not a predetermined constant but was instead determined by the moment when complete photodegradation of photosensitizer (PS) molecules occurred, known as the "photobleaching" effect. Once this occurred, the PS could no longer generate singlet oxygen and further irradiation was deemed ineffective and stopped. To monitor the fluorescence, the total duration of therapy was divided into sessions of 2-5 minutes, with breaks of 1-2 minutes for spectroscopic measurements to assess the fluorescence index (IF) – the ratio of the fluorescence intensity of the PS to the intensity of the backscattered laser signal. The therapy was considered complete when the characteristic red glow of the PS disappeared, resulting in a decrease in FI to levels like those found in normal tissues.
The light dose (J/cm2) was calculated after the completion of PDT using the formula:
Light dose = power density (mV/cm2) × therapy duration (s).
The power density was determined using the formula:
Power density = laser output power (1850 mV): irradiated area (cm2).
Safety protocols were strictly adhered to during the photodynamic therapy (PDT) procedure. This included continuously irrigating the removed tumor bed with cold physiological solution using a light guide immersed in it. To protect the personnel's eyes from the laser radiation, they wore glasses with filters that blocked the specific wavelength. After the PDT session, tissue samples were taken from the perifocal zone of the tumor for further detailed pathomorphological examination on permanent preparations. To prevent phototoxicity, the patient was advised to wear dark glasses for the next 24 hours to avoid direct sunlight on the retina. Additionally, all patients underwent the following post-PDT measures: a) monitoring of creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) levels; b) assessment of neurological status within 4 hours and over time; c) control of computed tomography and magnetic resonance imaging (CT/MRI) to evaluate the severity of perifocal edema; d) administration of hormonal decongestant therapy within 24 hours.
Histological examination
In the pathology department, the material was fixed in 10% buffered formalin, dehydrated in a standard manner and embedded in paraffin. Histological sections stained with hematoxylin and eosin were studied, as well as the results of immunohistochemical (IHC) reactions with rabbit polyclonal antibody to active caspase-3 by Merckmillipore, Darmstadt, Germany (PC679), 1:100. The EnVision polymer detection system (Dako, CA, USA) was also used. For visualization, the streptavidin–peroxidase polymer ultrasensitive system and DAB chromogen (Sigma-Aldrich, Darmstadt, Germany) were used. The sections were coun-terstained with Gill’s hematoxylin and then embedded in Bio Mount HM synthetic embedding medium (BIO-OPTICA, Milano, Italy). Additionally, reactions lacking primary antibodies were carried outto ensure the specificity of the observed staining. Histological analysis and microphotography were carried out using a Leica Aperio AT2 scanning microscope and Aperio Image Scope image manager (Leica Microsystems, USA). Quantification of the results of IHC reactions (positive staining of IHC cells in sections of the brain tissue, number) with antibodies to caspase-3 was performed by counting stained nuclei (×400) in sections in 1 mm2 (Image G).
Statistical Analysis
The Shapiro-Wilk test was used to assess the normality of distribution. Data are presented in the format M ± m (arithmetic mean ± standard error). Statistical analysis was performed using Microsoft Office Excel 2010 (WA, USA), Statistica v. 10 (Tibco, CA, USA). Differences between two samples with measured attributes were determined using non-parametric statistical methods (for example, the Mann–Whitney U test if at least one of the samples was not normal). When testing hypotheses for all criteria, the critical significance level was taken <0>
Ethics Statement
The study was conducted in accordance with the Helsinki Declaration of Human Rights. All patients (their representatives) signed informed voluntary consent to participate in the study. Preoperative examination and surgical treatment of patients were carried out in accordance with the Clinical Guidelines of the Association of Neurosurgeons of Russia 2015.
Results
When examining the histological material from all patients, it was confirmed that they had metastatic brain damage. The most common types of metastases found in the tumors studied were colon adenocarcinoma (6 cases, 24%) and squamous cell lung carcinoma (6 cases, 24%) (Figure 1 A-B). The study also included 4 cases (16%) of metastatic breast carcinoma (Figure 1C), 3 cases of metastatic clear cell renal cell carcinoma (12%) (Figure 1D), 2 cases each of neuroendocrine small cell lung carcinoma (8%) and melanoma (8%), and 1 case each of metastasis from squamous cell carcinoma of the cervix (4%) and adenocarcinoma of the lung (4%).
Figure 1: Results of histological examination of resected brain metastases, H&E stain. A-Metastatic growth of colon adenocarcinoma, ×200; B-Metastatic growth of squamous cell carcinoma of the lung, ×100; C-Metastatic growth of breast carcinoma, ×200; D-Metastatic growth of clear cell renal cell carcinoma, ×200.
In 22 patients, tissue fragments labeled as "Cortex over tumor" were present. A histological examination of these fragments from the cerebral cortex above the tumor revealed several changes, including pericellular edema, pronounced vascular congestion, gliosis, accumulation of lipofuscin in the cytoplasm of neurons, and the phenomenon of neuronal satellitetosis (Figure 2A). In an immunohistochemical study, caspase-3 expression was predominantly detected in oligodendrogliocytes. The number of positive cells in patients with brain metastatic lesions in the cerebral cortex ranged from 0 to 38, compared to 0 to 1 in the comparison group (Figure 2B-D). The data, presented in arithmetic mean format with their standard deviation, are shown in Table 1. Statistical analysis using the Mann-Whitney U test showed a significant difference (p less than 0.05) (Figure 6A).
Figure 2: Results of histological and immunohistochemical examination of the cortex area above the tumor. A-Dystrophic changes in neurons, gliosis, edema and plethora in the cerebral cortex. H&E stain, ×200; B-Low level of caspase-3 expression in cortical glial cells. IHC, ×400; C-High level of caspase-3 expression in cortical glial cells. IHC, ×400; D-Absence of nuclear expression of caspase-3 in gliocytes from a patient in the comparison group. IHC, ×400
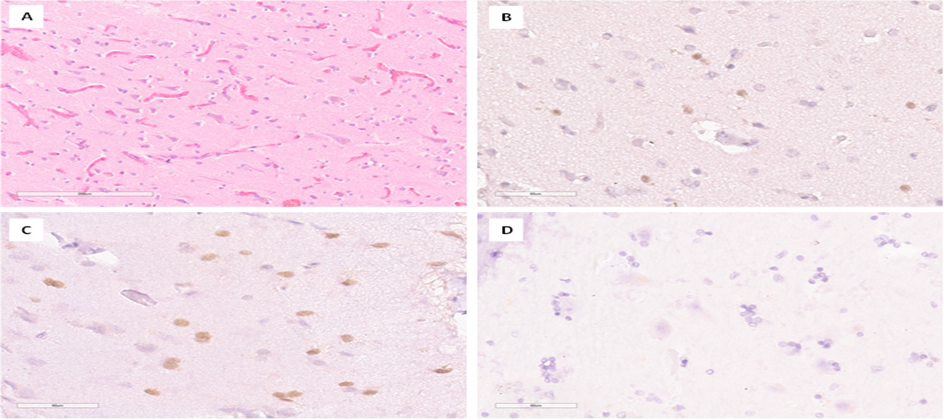
In 24 patients, tissue fragments labeled as "Perifocal tumor zone" were present. Histological examination of fragments of brain matters revealed structures of the cortex and white matter. The tissue revealed pericellular edema, vascular congestion, gliosis, accumulation of lipofuscin in the cytoplasm of neurons, numerous shadow cells, and phenomena of neuronal satellitetosis (Figure 3A). In an IHC study, caspase-3 expression was predominantly detected in oligodendrogliocytes. The number (n) of positive cells in patients with brain metastatic lesions in the perifocal tumor zone ranged from 0 to 38, compared to 0 in the comparison group (Figure 3B-D). The data, presented in arithmetic mean format with their standard deviation, are shown in Table 1. Statistical analysis using the Mann-Whitney U test showed a significant difference (p less than 0.05) (Figure 6B).
Figure 3:Results of histological and immunohistochemical examination of the perifocal zone of the tumor. A-Dystrophic changes in neurons, gliosis, edema and plethora in the brain substance. H&E stain, ×100; B-Low level of caspase-3 expression in glial cells. IHC, ×400; C-High level of caspase-3 expression in glial cells. IHC, ×400; D-Absence of nuclear expression of caspase-3 in gliocytes from a patient in the comparison group. IHC, ×400
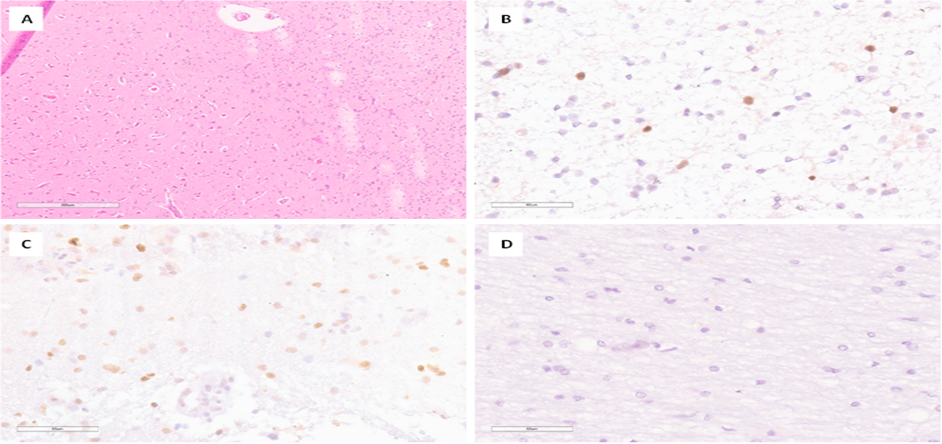
In 8 patients, tissue fragments labeled as "Perifocal tumor 1 cm from tumor" were present. Histological examination of fragments of the brain substance revealed white matter with pronounced gliosis and the presence of reactive “fat” astrocytes; pericellular edema and vascular congestion were noted (Figure 4A). In immunohistochemical study, caspase-3 expression was detected predominantly in oligodendrogliocytes, the number (n) of positive cells in patients with brain metastatic lesions in this area ranged from 0 to 40, compared 0 in the comparison group (Figure 4B-D). The data, presented in arithmetic mean format with their standard deviation, are shown in Table 1. Statistical analysis using the Mann-Whitney U test showed a significant difference (p less than 0.05) (Figure 6C).
Figure 4: Results of histological and immunohistochemical examination of the perifocal zone 1 cm from the tumor.
A – White matter gliosis with the presence of reactive astrocytes (indicated by an arrow). H&E stain, ×100; B-Low level of caspase-3 expression in glial cells. IHC, ×400; C-High level of caspase-3 expression in glial cells. IHC, ×400; D-Absence of nuclear expression of caspase-3 in gliocytes from a patient in the comparison group. IHC, ×400
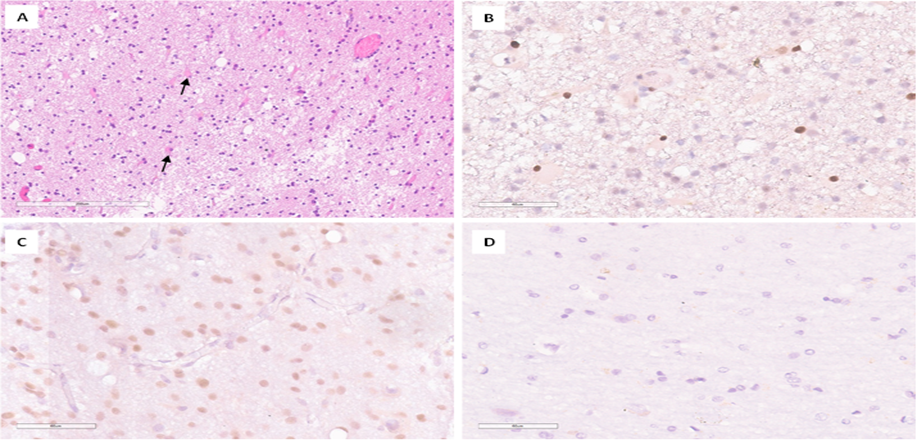
In 9 patients, tissue fragments labeled as " Perifocal zone after PDT " were present. Upon histological examination, these fragments showed white matter with pronounced gliosis, the presence of reactive astrocytes, rarefaction of the neuropil with loss of fibrillarity, and the formation of microcysts. Additionally, pericellular edema, vascular congestion, and perivascular accumulations of gliocytes were observed (see Figure 5A). The nuclei of cells showed hyperchromatosis. In caspase-3 IHC, the number (n) of positive cells in patients with brain metastases in the cerebral cortex ranged from 4 to 212, compared to 0 in the control group (Figure 5B-D). Furthermore, in most patients, the number of positive gliocyte nuclei varied from 4 to 48. However, in one patient, these numbers were significantly higher, ranging from 137 to 212 cells (with an arithmetic mean of 160.2). The data, presented in arithmetic mean format and their standard deviation, are shown in Table 1. Statistical analysis using the Mann-Whitney U test showed a significant difference (p less than 0.05) (Figure 6D).
Figure 5: Results of histological and immunohistochemical examination of the perifocal zone after intraoperative photodynamic therapy. A-White matter gliosis with rarefaction of the neuropil and the presence of reactive astrocytes (indicated by an arrow). H&E stain, ×200; B-Low level of caspase-3 expression in glial cells, predominantly in reactive astrocytes. IHC, ×400; C-High level of caspase-3 expression in glial cells. IHC, ×400; D-Absence of nuclear expression of caspase-3 in gliocytes from a patient in the comparison group. IHC, ×400
Table 1: The level of expression of caspase-3 (number) in the brain structures of patients with metastases and the comparison group M ± m, p-value result (Mann–Whitney U test).
| Region | Caspase 3, n. | p-Value | |
| Patients with brain metastases | Comparison group | ||
| Сerebral cortex above the tumor | 11.9 ± 5.81 | 0.005 ± 0.2 | p<0> |
| Perifocal tumor zone | 12.7 ± 9.46 | 0.12 ± 0.3 | p<0> |
| Perifocal zone 1 cm from the tumor | 9.1 ± 8.58 | 0 ± 0 | p<0> |
| Perifocal zone after PDT | 35.2 ± 48.6 | 0 ± 0 | p<0> |
Figure 6: Range diagram of data caspase-3 expression in the Cortex above the tumor (A), Perifocal tumor zone (B), Perifocal zone 1 cm from the tumor (C) and Perifocal zone after PDT (D) (explanation in the text). Group 1 – patients with brain metastases, O – patients of the comparison group.
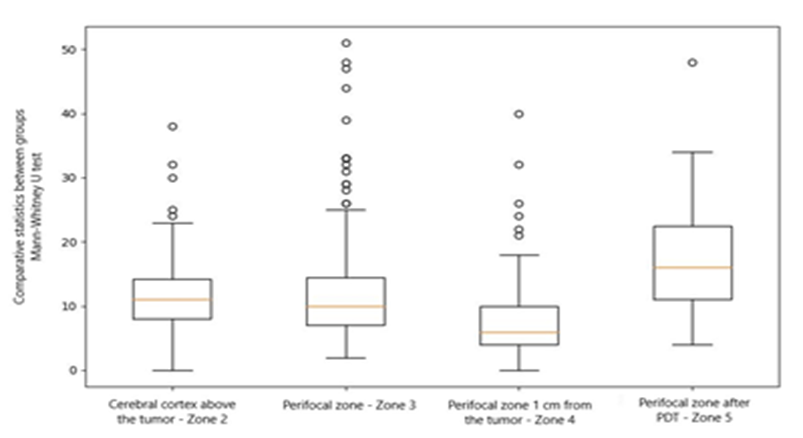
When analyzing the data statistically, we found significant differences between the cortex zone above the tumor and the perifocal zone after PDT (p = 0.0005). Additionally, there were significant differences between the perifocal zone and the perifocal zone 1 cm from the tumor (p = 0.001), as determined by the Mann-Whitney U test (see Table 2 and Figure 7).
Table 2: Results of comparative statistics between groups, p-value result (Mann–Whitney U test)
| Region | Сerebral cortex above the tumor | Perifocal tumor zone | Perifocal zone 1 cm from the tumor | Perifocal zone after PDT |
| Сerebral cortex above the tumor | 1.0 | 0.25 | 4.45 | 0.0005 |
| Perifocal tumor zone | 0.25 | 1.0 | 0.001 | 1.39 |
| Perifocal zone 1 cm from the tumor | 4.45 | 0.001 | 1.0 | 0 |
| Perifocal zone after PDT | 0.0005 | 1.39 | 0 | 1.0 |
Figure 7: Range diagram of data caspase-3 expression in all studied areas.
When analyzing the data, it was observed that there was a decrease in apoptotic activity of gliocytes located 1 cm from the tumor, followed by an increase after PDT (p less than 0.05). However, a correlation analysis using Spearman's rank correlation did not yield any significant differences.
Discussion
Previous studies have demonstrated the role of astrocytes in promoting cancer cell proliferation through the release of proinflammatory cytokines [19]. Additionally, microglial cells have been found to play an active role in brain metastases [20-21] and targeting the colony stimulating factor 1 receptor (CSF-1R) has been shown to reduce both the size and number of brain metastases in an experimental model of melanoma [21]. Furthermore, activated microglia can regulate the invasion and colonization of the brain by metastasizing cancer cells through the WNT pathway [22]. However, the role of oligodendrocytes in the development of metastatic brain carcinomas has not been established, even though these cells make up more than 60% of all brain cells. In our study, we found that oligodendrocytes were the primary cells affected, with astrocytes only being affected by PDT. It is well known that the main function of oligodendrocytes is to produce myelin. This is important because myelin is metabolically active and contains pathways for the movement of macromolecules into the periaxonal space. The myelin sheath and the adjacent axon should be considered as one functional unit, as they are not only connected morphologically but also metabolically [23]. However, it is unknown whether macromolecules are exchanged between neurons and glia. The extent of myelin sheath formation may also serve as a form of plasticity to adapt brain function to environmental stimuli, which are metastases [24–25].
PDT involves the interaction of light, a photosensitizing agent, and molecular oxygen [26-27]. When cells are irradiated, reactive oxygen species can be generated at subcellular sites where the photosensitizer has accumulated, causing fatal damage [28]. It is known that photosensitizing agents can be targeted at different cell organelles, such as mitochondria and lysosomes. For example, Yousefi et al. demonstrated the multiple roles of autophagy-related protein (ATG) in cell death during PDT. The release of Ca2+ from lysosomes into the cytoplasm activates calpain, which then cleaves the ATG5 protein, resulting in the formation of a truncated molecule (tATG5) that can bind to mitochondria and promote apoptosis [29]. According to a recent report, targeting both the Golgi and lysosomes simultaneously may contribute to apoptotic death induced by PDT, although the specific mechanism is still unknown [30]. This finding supports the use of PDT to target both lysosomes and mitochondria to effectively eradicate tumors [31]. In a study by Pevna et al, it was observed that the switch between autophagy and apoptosis was dependent on the dosage and occurred independently of photobiomodulation (PBM) in human dermal fibroblasts treated with hypericin-mediated PDT. PBM was found to preferentially induce autophagy in non-cancerous cells, potentially allowing them to escape apoptosis under certain conditions [32]. Similarly, in a study by Yao Y et al, increased activation of caspase-2, caspase-9, and tumor necrosis factor was observed, along with decreased activity of bcl2, prkn, atg2, atg9, and atg10. The researchers also found that PDT using Elsinochrome A induced cell apoptosis and autophagy through the ROS/Atg/Parkin pathway [33]. It is possible that similar mechanisms may be activated in the glial cells surrounding brain metastases.
In a single case, a patient with metastatic squamous cell carcinoma showed a 10-fold increase in apoptotic activity after undergoing PDT. Additionally, the initial level of apoptosis in gliocytes in the perifocal zone was also found to be high. This suggests that not only the photosensitizing drug, but also the type of malignant tumor and its microenvironment may play a role in the death of gliocytes.
Conclusion
In the perifocal zone of metastatic brain tumors, there is an increase in gliocyte apoptosis with a decrease in activity towards the periphery. PDT using Photoditazine significantly increases the activity of apoptosis in glial cells. Hypoxia, metabolic remodeling, and neuroinflammation-induced brain tissue remodeling likely contribute to tumor propagation. At the same time, the influence of gliocyte apoptosis on the relapse-free survival of patients with brain metastases, as well as the induction/inhibition of caspase-dependent apoptosis mechanisms may be a potential therapeutic target in the complex treatment of metastatic carcinomas.
Declarations
Conflicts of Interest
The authors declare no conflict of interest.
Funding
This study is part of the state assignment of the Ministry of Health of the Russian Federation of Almazov National Medical Research Centre (ANMRC) No 123021000128-4.
Institutional Review Board Statement
The work was carried out according to the principles of voluntariness and confidentiality in accordance with Federal Law “On the Basics of Health Protection of Citizens in Russian Federation” 21.11.2011 N 323-FZ, and the Helsinki Declaration on Human Rights, and approved by the ethical committee of Almazov National Medical Research Centre (ANMRC), St. Petersburg, Russia. Informed Consent Statement Written consent of the subjects is available.
References
- Yuzhalin AE, Yu D. (2020). Brain Metastasis Organotropism. Cold Spring Harb Perspect Med, 10(5):a037242.
Publisher | Google Scholor - Boire A, Brastianos PK, Garzia L, Valiente M. (2020). Brain metastasis. Nat Rev Cancer, 20(1):4-11.
Publisher | Google Scholor - Sklyar SS, Safarov BI, Trashkov AP, Zorina E.Y. et al. Solid tumors’ metastasis mechanisms into the brain // Pediatrician (St. Petersburg), 14(6):59-69.
Publisher | Google Scholor - Akimoto J. (2016). Photodynamic Therapy for Malignant Brain Tumors. Neurol Med Chir (Tokyo), 56(4):151-157.
Publisher | Google Scholor - Naidoo C, Kruger CA, Abrahamse H. (2018). Photodynamic Therapy for Metastatic Melanoma Treatment: A Review. Technol Cancer Res Treat, 17:1533033818791795.
Publisher | Google Scholor - Cen J, Huang Y, Liu J, Liu Y. (2022). Thermo-responsive palladium-ruthenium nanozyme synergistic photodynamic therapy for metastatic breast cancer management. J Mater Chem B, 10(48):10027-10041.
Publisher | Google Scholor - Rynda AYu, Olyushin VE, Rostovtsev DM, Zabrodskaya YM et al. (2021). Fluorescent diagnostics with chlorin e6 in surgery of low-grade glioma. Biomedical Photonics, 10(4):35-43.
Publisher | Google Scholor - Rafaelian A, Martynov B, Chemodakova K, Martynov RS et al. (2023). Photodynamic interstitial stereotactic therapy for recurrent malignant glioma. Asian J Oncol, 9:14.
Publisher | Google Scholor - Lowery FJ, Yu D. (2017). Brain metastasis: Unique challenges and open opportunities. Biochim Biophys Acta Rev Cancer, 1867(1):49-57.
Publisher | Google Scholor - Costa-Silva B, Aiello NM, Ocean AJ, Singh S et al. (2015). Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol, 17(6):816-826.
Publisher | Google Scholor - Liu Y, Cao X. (2016). Characteristics and Significance of the Pre-metastatic Niche. Cancer Cell, 30(5):668-681.
Publisher | Google Scholor - Paget S. (1989). The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev, 8(2):98-101
Publisher | Google Scholor - Budczies J, von Winterfeld M, Klauschen F, Bockmayr M et al. (2015). The landscape of metastatic progression patterns across major human cancers. Oncotarget, 6(1):570-583.
Publisher | Google Scholor - Obenauf AC, Massagué J. (2015). Surviving at a Distance: Organ-Specific Metastasis. Trends Cancer, 1(1):76-91.
Publisher | Google Scholor - Wilhelm I, Molnár J, Fazakas C, Haskó J, Krizbai IA. (2013). Role of the blood-brain barrier in the formation of brain metastases. Int J Mol Sci, 14(1):1383-1411.
Publisher | Google Scholor - Schwartz H, Blacher E, Amer M, Livneh N et al. (2016). Incipient Melanoma Brain Metastases Instigate Astrogliosis and Neuroinflammation. Cancer Res, 76(15):4359-4371.
Publisher | Google Scholor - Fidler IJ. (2011). The role of the organ microenvironment in brain metastasis. Semin Cancer Biol. 21(2):107-112.
Publisher | Google Scholor - Sokolova TV, Zabrodskaya YM, Litovchenko AV, Paramonova NM et al. (2022). Relationship between Neuroglial Apoptosis and Neuroinflammation in the Epileptic Focus of the Brain and in the Blood of Patients with Drug-Resistant Epilepsy. Int J Mol Sci, 23(20):12561.
Publisher | Google Scholor - Sitovskaya D, Zabrodskaya Y, Parshakov P, Sokolova T et al. (2023). Expression of Cytoskeletal Proteins (GFAP, Vimentin), Proapoptotic Protein (Caspase-3) and Protective Protein (S100) in the Epileptic Focus in Adults and Children with Drug-Resistant Temporal Lobe Epilepsy Associated with Focal Cortical Dysplasia. Int J Mol Sci, 24(19):14490.
Publisher | Google Scholor - Seike T, Fujita K, Yamakawa Y, Kido MA et al. (2011). Interaction between lung cancer cells and astrocytes via specific inflammatory cytokines in the microenvironment of brain metastasis. Clin Exp Metastasis, 28(1):13-25.
Publisher | Google Scholor - Bowman RL, Klemm F, Akkari L, Pyonteck SM et al. (2016). Macrophage Ontogeny Underlies Differences in Tumor-Specific Education in Brain Malignancies. Cell Rep, 17(9):2445-2459.
Publisher | Google Scholor - Qiao S, Qian Y, Xu G, Luo Q, Zhang Z. (2019). Long-term characterization of activated microglia/macrophages facilitating the development of experimental brain metastasis through intravital microscopic imaging. J Neuroinflammation, 6(1):4.
Publisher | Google Scholor - Pukrop T, Dehghani F, Chuang HN, Lohaus R et al. (2010). Microglia promote colonization of brain tissue by breast cancer cells in a Wnt-dependent way. Glia, 58(12):1477-1489.
Publisher | Google Scholor - Simons M, Nave KA. (2015). Oligodendrocytes: Myelination and Axonal Support. Cold Spring Harb Perspect Biol, 8(1):a020479.
Publisher | Google Scholor - Makinodan M, Rosen KM, Ito S, Corfas G. (2012). A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science, 337(6100):1357-1360.
Publisher | Google Scholor - Young KM, Psachoulia K, Tripathi RB, Dunn SJ et al. (2013). Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron, 77(5):873-885.
Publisher | Google Scholor - Cengel KA, Simone CB 2nd, Glatstein E. (2016). PDT: What's Past Is Prologue. Cancer Res, 76(9):2497-2499.
Publisher | Google Scholor - Kukanov KK, Nechaeva AS, Sitovskaya DA, Dikonenko MV et al. Intraoperative photodynamic therapy in the structure of complex treatment of patients suffering from recurrence and continued growth of intracranial meningioma. Bulletin of the Russian Military Medical Academy, 26(2):243-2458.
Publisher | Google Scholor - Kessel D, Reiners JJ. (2020). Photodynamic therapy: autophagy and mitophagy, apoptosis and paraptosis. Autophagy, 16(11):2098-2101.
Publisher | Google Scholor - Yousefi S, Perozzo R, Schmid I, Ziemiecki A et al. (2006). Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol, 8(10):1124-1132.
Publisher | Google Scholor - Villanueva A, Stockert JC, Cañete M, Acedo P. (2010). A new protocol in photodynamic therapy: enhanced tumour cell death by combining two different photosensitizers. Photochem Photobiol Sci, 9(3):295-297.
Publisher | Google Scholor - Plaetzer K, Kiesslich T, Oberdanner CB, Krammer B. (2005). Apoptosis following photodynamic tumor therapy: induction, mechanisms and detection. Curr Pharm Des, 11(9):1151-1165.
Publisher | Google Scholor - Pevna V, Horvath D, Wagnieres G, Huntosova V. (2022). Photobiomodulation and photodynamic therapy-induced switching of autophagy and apoptosis in human dermal fibroblasts. J Photochem Photobiol B, 234:112539.
Publisher | Google Scholor - Yao Y, Pan L, Song W, Yuan Y et al. (2023). Elsinochrome A induces cell apoptosis and autophagy in photodynamic therapy. J Cell Biochem, 124(9):1346-1365.
Publisher | Google Scholor