Research article
Synthesis, Characterization and Antimicrobial Activity of Copper Oxide Nanoparticles Using Sol-Gel Method
- Jagannath S. Godse 1
- Kishore Puri 2
- Santosh B. Gaikwad 3
- Sanjay B. Ubale 1
- Savita A. Kate 4
- Rajendra P. Pawar 4
1Department of Chemistry, Deogiri College, Aurangabad, India.
2Department of Chemistry, Shri Shivaji College of Arts, Commerce and Science, Akola, India.
3Department of Chemistry, L P G Arts and Science College, Shirpur (Jain), Washim, India.
4Department of Chemistry, Shi Chhatrapati College, Aurangabad, India.
*Corresponding Author: Sanjay B. Ubale, Department of Chemistry, Deogiri College, Aurangabad, India; Rajendra P. Pawar, Department of Chemistry, Shi Chhatrapati College, Aurangabad, India
Citation: Rajendra P. Pawar, Kishore Puri, Santosh B. Gaikwad, Sanjay B. Ubale, Savita A. (2023). Synthesis, Characterization and Antimicrobial Activity of Copper Oxide Nanoparticles Using Sol-Gel Method, Clinical Interventions and Clinical Trials, BRS Publishers. 1(1); DOI: 10.59657/2993-1096.brs.23.002
Copyright: © 2023 Rajendra P. Pawar and Sanjay B. Ubale, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: April 20, 2023 | Accepted: May 09, 2023 | Published: May 15, 2023
Abstract
This study investigates a novel way for producing copper oxide (CuO) nanoparticles using the sol-gel process and citric acid as a surfactant. In this technique, water is used as a potent solvent. Because of its low cost, ease of preparation, and commercial practicality, the synthetic approach is environmentally beneficial. X-ray diffraction (XRD), Field Emission Scanning Electron Microscopy (FE-SEM), Energy-Dispersive X-ray spectroscopy (EDX), Fourier Transform Infra-Red spectroscopy (FTIR), and Ultra-Violet visible spectroscopy (UV-Vis) were used to examine the antimicrobial activity of bacterial, fungal, as well as Pneumonia activity. Copper oxide NPs synthesized demonstrates highly scattered characterization and application findings. The green method is a practical, eco-friendly, simple, and valuable method for producing copper oxide nanoparticles
Keywords: copper oxide; sol-gel method; nanomaterial; antimicrobial; antifungal
Introduction
The unique properties of nanomaterials, as well as their high surface-to-volume ratio, have made them a viable and new tool in many scientific disciplines. This flexible material has numerous applications, including antimicrobial activities, photovoltaics, sensors, storage devices, medicine administration, photocatalytic training, and so on. Copper oxide nanoparticles most used because they are cheap, common, have low toxicity, and are easy to manufacture. Because of their high catalytic activity and durability, photo-catalysts constructed of single metal oxides are becoming increasingly popular [1-2].
A multitude of procedures can be used to create single metal oxide nanoparticles. Solid-state synthesis, the hydrothermal method, the electrochemical approach, the sol-gel technique, co-precipitation, microwave-assisted synthesis, the thermal decomposition method, and others are examples[3-4]. We choose the sol-gel process due to worries about overheating and hazardous solvents in other procedures. The sol-gel method is the most environmentally friendly and cutting-edge of these techniques because it does not require high pressure or high temperature, is inexpensive, and allows for easy monitoring of the crystalline size and structure of the nanomaterial through pH modification of the medium while preparing a large amount of sample. This paper describes a simple sol-gel method for producing copper oxide (CuO) nanoparticles with citric acid as a suitable surfactant. Synthesized Copper oxide nanoparticles were screened for biological activity of antibacterial, antifungal, and antimalarial activity which showed better to excellent results.
Introduction
The unique properties of nanomaterials, as well as their high surface-to-volume ratio, have made them a viable and new tool in many scientific disciplines. This flexible material has numerous applications, including antimicrobial activities, photovoltaics, sensors, storage devices, medicine administration, photocatalytic training, and so on. Copper oxide nanoparticles most used because they are cheap, common, have low toxicity, and are easy to manufacture. Because of their high catalytic activity and durability, photo-catalysts constructed of single metal oxides are becoming increasingly popular [1-2].
A multitude of procedures can be used to create single metal oxide nanoparticles. Solid-state synthesis, the hydrothermal method, the electrochemical approach, the sol-gel technique, co-precipitation, microwave-assisted synthesis, the thermal decomposition method, and others are examples[3-4]. We choose the sol-gel process due to worries about overheating and hazardous solvents in other procedures. The sol-gel method is the most environmentally friendly and cutting-edge of these techniques because it does not require high pressure or high temperature, is inexpensive, and allows for easy monitoring of the crystalline size and structure of the nanomaterial through pH modification of the medium while preparing a large amount of sample. This paper describes a simple sol-gel method for producing copper oxide (CuO) nanoparticles with citric acid as a suitable surfactant. Synthesized Copper oxide nanoparticles were screened for biological activity of antibacterial, antifungal, and antimalarial activity which showed better to excellent results.
Experimental
Characterization
Copper chloride, citric acid, hydrochloric acid, and ammonium hydroxide were purchased from SD-Fine Chemical Company and used without further purification. The X-ray diffraction (XRD) pattern was recorded by the Rigaku X-ray Diffractometer with a Cu K-α radiation source (λ=1.5406 A°) operated at 40 kV. Field emission scanning electron microscopy (FE-SEM) images were obtained on JEOL 6390LA, Japanand the energy dispersive spectrometry (EDS) analysis was studied by JEOL/JEM 2100, Japan, with an acceleration voltage of 200 kV. Fourier transform infrared (FT-IR) spectra were recorded on Shimadzu IR spectrophotometer. Ultraviolet visible (UV-Vis) spectra were recorded on Thermo Scientific UV-Vis’s spectrophotometer.
Synthesis of CuMn2O4 nanoparticles
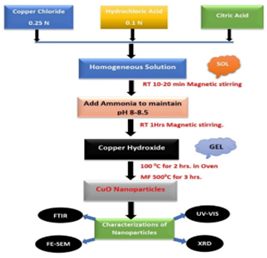
Synthesis of Copper oxide (CuO) nanoparticles was carried out by the sol-gel method. Initially, 0.25 M of copper chloride were dissolved in 100 ml of distilled water. 0.1 N hydrochloric acid improves the solubility of copper chloride in water. Both the solutions were mixed with constant stirring. After mixingthe solutions, 1 gm of the citric acid solution was added as a surfactant. The pH of the above solution was adjusted at 8-8.5 (basic) using 3 to 3.5 ml NH4OH solution. Precipitation was formed, which stirred with constant stirring for an hour at room temperature. The mixture was filtered using Whatman filter paper No. 42 and washed with deionized water 2-3 times. The obtained product was dried in an oven at 100˚C for 2 hours. Finally, the dried material was calcinated for 3 hours at 500˚C in the furnace. The black-colored precipitate was obtained as a final product and was stored for further study (Figure 1).
Figure 1: Flow Chart for Synthesis Method of CuO NPs
Result and Discussion
UV-Visible Analysis of CuO oxide Nanoparticle
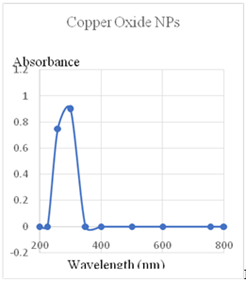
The UV-Visible diffused reflectance spectra of nanostructured equimolar concentration of copper oxide NPs are shown in Figure. It has a wide range of absorption at 260 to 300 nm [5]. This proves that equimolar concentrations of copper oxide NPs result in lower reflectance. These findings suggest that synthesized copper oxide NPs metal to shift the absorption of the resulting nanoparticles from the infrared to the visible. This indicates that transition metal oxides that have been optically active.
The copper oxide NPs effect on the band gaps of CuO semiconductors is the more immediate cause of the change in reflectance. The increased photogeneration rate of charge-transfer between Cu2+ electrons valence band greatly improves the photo reactivity, catalytic activity of copper oxide NPs (Figure 2).
Figure 2: Uv-Vis’s data of CuO NPs
FTIR analysis of CuO oxide nanomaterial
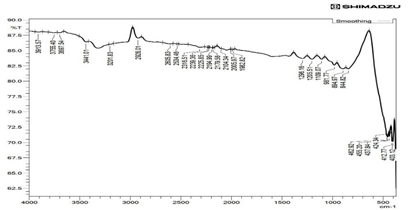
Fourier transform infrared spectrometer confirms the formation of metal oxide bonds in the CuO nanoparticles as shown in Figure 2. Investigation of nanomaterial is observed at the wavelength range from 397 cm-1 to 3980 cm-1. The low-frequency vibrations of the M-O bond appear below 1000 cm-1. In Figure 1the firm peaks at position 403.12cm-1 and 462.92 cm-1 represent Cu–O stretching of CuO stretching vibrational modes, respectively and the band that appeared in the range of 800–1300 cm-1 is recognized as the stretching vibration of –OH (Figure 3). These results as approximately match with another study [6-7].
Figure 3: FTIR data of CuO NPs
XRD characterization of CuMn2O4 oxide nanomaterial
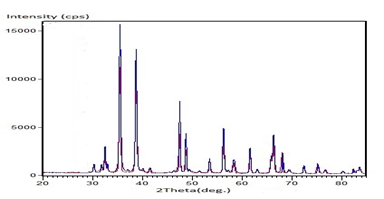
The X-ray diffraction technique investigated the chemical, structural, and spectroscopic properties of CuO nanomaterial. All X-ray diffraction peaks were indexed according to the standard data for CuO as shown in Figure 2. The significant peaks are obtained for 2θ at 32.5°,35.49°, 38.71°, 47.42°, 56.29°, 61.59°, 66.32068.11°and 75.17°, corresponding to the planes (110), (111), (111), (220), (020), (113), (022) (220) and (004) respectively (Figure 4). In addition, the Fd-3m space group and Monoclinic Crystal system of CuO nanomaterial are recognized in the X-ray diffraction pattern and correctly matched with the standard JCPDs card no. 05-0661[8-9]. The average crystallite size (D) of CuO nanomaterial was calculated by using the Scherer formula [5, 10].
Where, K is a shape factor, D is the size of the nanomaterial, λ is the incident X-ray beam’s wave length, θ is Bragg’s diffraction angle, and β is the Full width half maximum. The average particle size of the investigating nanomaterial was 15.5 nm.
Figure 4: XRD data of CuO NPs
SEM and EDX studies of CuO oxide nanomaterial
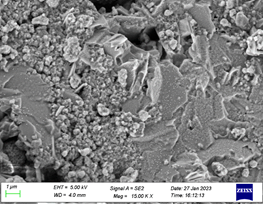
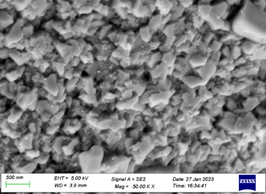
The morphology and complete structures of CuO nanomaterial were contributed by scanning electron microscope (SEM) and energy-dispersive X-ray (EDX) spectroscopy. SEM images of CuO nanomaterial synthesized by sol-gel method from chloride precursors strengthening the precursors at 500°C are shown in as shown in (Figure 6 and Figure 7). Scanning electron microscope (SEM) instrument in the range of scale taken between 500 nm and 1μm.
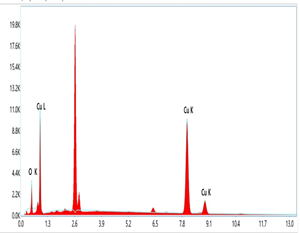
The elemental compositional investigation of CuO nanomaterial is shown in as shown in (Figure 8 and Table 1). The presence of Cu and O and the Cu/O ratio were determined by EDX investigation, which matches perfectly with CuO. This confirms the availability of copper and oxygen with a weight percentage of 94 %, 6 %, and atomic percentages of 79.9% and 20.1% respectively.
Figure 5: FESEM data of CuO NPs
Figure 6: FESEM data of CuO NPs
Figure 7: EDX data of CuO NPs
Table 1: EDX data of CuO NPs
| Element | Line Type | Wt.% | Atomic % |
| O | K series | 6 | 20.1 |
| Cu | K series | 94 | 79.9 |
| Total: | 100 | 100 |
In recent years, nanomaterial has a broader range of applications and uses in various fields. Some researchers have studied the use of nanomaterials for catalytic activity for oxidation [11], photo-catalytic activity [12], a high-performance electrode for supercapacitors [13], solid oxide fuel cells [14]and catalytic ozonation membrane reactor [15]. These example sconfirm that CuO oxide nanomaterial is environmentally supportive for living organisms and extremely useful in different fields.
Biological Activity
Synthesized copper oxide nanoparticles were tested for their antimicrobial efficacy against four different bacteria. - S. aureus (MTCC 96), E. coli (MTCC 443), P. aeruginosa (MTCC 1688), S. pyogenus (MTCC442), and three fungi-C. Albicans (MTCC 227), A. clavatus (MTCC1323)and A. niger (MTCC282 )using broth dilution method[16-17]. Gentamycin, Ampicillin chloramphenicol, ciprofloxacin and norfloxacin were used as the standards for antibacterial activity, whereas griseofulvin and nystatin were used as the standards for antifungal activity. Minimum Inhibitory Concentration (MIC) values were used to describe the outcomes. The MIC of a drug is the smallest concentration of the sample that clearly stunts the growth of the microbe. Minimum Inhibitory Concentration (MIC) values were used to describe the outcomes. The MIC of a drug is the smallest concentration of the sample that clearly stunts the growth of the microbe[18-19].
The efficiency of synthesized copper oxide nanoparticles to act as antimalarial hits was studied in-vitro against Plasmodium falciparum in 96-well microtitre plate according to the different reported method [20-21].chloroquine was used as the standard, and the results were reported in terms of IC50 values. All the screening was performed at Microcare Laboratory, Surat, Gujarat, India.
Antibacterial activity
Antibacterial activity was tested on the synthesized copper oxide nanoparticles compounds[22-23]. The broth dilution technique was used to demonstrate antibacterial activity against E. coli (MTCC443), P. aeruginosa (MTCC1688), S. aureus (MTCC96), S. pyogenus (MTCC442) bacterial strains using Gentamycin, Ampicillin, chloramphenicol, ciprofloxacin and norfloxacin as the standard drugs. Copper oxide nanoparticles showing a strong and excellent antibacterial activity against S. aureus. The standard chloramphenicol (50µg/mL) other had weaker antibacterial activity against E. coli, P. aeruginosa and S. pyogenus as compared to the standard chloramphenicol show in (Table1). copper oxide nanoparticles showing a weak antibacterial activity against E. coli, P. aeruginosa, S. aureus and S. pyogenus the standard Gentamycin, Ampicillin, ciprofloxacin and norfloxacin show in (Table 2).
Table 2: Antibacterial activity data of CuO NPs
| Antibacterial Activity Table | |||||
| Minimum Inhibition Concentration (Microgram /ML) | |||||
| SR. | Code | E. Coli | P. Aeruginosa | S. Aureus | S. Pyogenus |
| NO | NO | MTCC 443 | MTCC 1688 | MTCC 96 | MTCC 442 |
| 1 | Sample CuO NPs | 250 | 50 | 100 | 100 |
| 2 | *Gentamycin | 0.05 | 1 | 0.25 | 0.5 |
| 3 | *Ampicillin | 30 | - | 40 | 25 |
| 4 | *Chloramphenicol | 50 | 50 | 50 | 50 |
| 5 | *Ciprofloxacin | 25 | 25 | 50 | 50 |
| 6 | *Norfloxacin | 10 | 10 | 10 | 10 |
Antifungal Activity
Antifungal Activity was studied against C. albicans (MTCC227), A. niger (MTCC282), A. clavatus (MTCC1323) fungal strains. The results of antifungal activity reveal that the copper oxide nanoparticles synthesized compounds showed lower antifungal activity as compared to the standard Nystatin and Greseofulvin. The compounds were less active against the C. albicans, A. niger and A. clavatus fungal strains in comparison with both the standards (Table 3).
Table 3: Antifungal activity data of CuO NPs
| Antifungal Activity Table | ||||
| Minimum Inhibition Concentration (Microgram /ML) | ||||
| SR. | Code | C. Albicans | A. Niger | A. Clavatus |
| NO | NO | MTCC 227 | MTCC 282 | MTCC 1323 |
| 1 | Sample CuO NPs | >1000 | 500 | 500 |
| 2 | *Nystatin | 100 | 100 | 100 |
| 3 | *Greseofulvin | 500 | 100 | 100 |
| *Standard Drug | ||||
Antimalarial Activity
Copper oxide nanoparticles synthesized compounds showed the antimalarial activity was carried against Plasmodium falciparum. The compound copper oxide nanoparticles showed less antimalarial activity. The results of antimalarial activity revealed that copper oxide nano particles compounds were moderately active but less potent than the standard chloroquine, which is show in (Table 4).
Table 4: Antimalarial activity data of CuO NPs
| Antimalarial Activity (Plasmodium Falciparum) | ||
| Minimum Inhibition Concentration (Microgram /ML) | ||
| SR. | Code | Mean IC50 Values a |
| NO | NO | MTCC 227 |
| 1 | Sample CuO NPs | 0.88μg /ml |
| 2 | *Chloroquine IC50 | 0.020 μg /ml |
| *Standard Drug, a: mean values in representative assay. | ||
Conclusion
In summary, we report the synthesis, characterization, antibacterial, antifungal and antimalarial activity of copper oxide nano particles antibacterial activity against E. coli as they had lower MIC values in comparison with the standard ampicillin. Copper oxide nanoparticles compounds the compounds were less active against the C. albicans, A. niger and A. clavatus fungal strains in comparison with both the standards. The compounds exhibited moderate antimalarial activity but less than the standard chloroquine. Antibacterial and antimalarial entities are thus revealed by the current studies, highlighting their potential future significance in medicinal chemistry. A CuO oxide nanomaterial was successfully prepared using a sol-gel, an environmentally supportive method. In the synthesis of CuO nanomaterial, copper chloride with using citric acid as a surfactant. This method is eco-friendly, cheap, easy handling, non-hazardous, and harmless aqueous solvent systems.
Declarations
Funding
This research received no external funding.
Acknowledgments
This research has no acknowledgment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ananda Murthy, H. Abebe, B. CH, P. Shantaveerayya, K. (2018). A review on green synthesis and applications of Cu and CuO nanoparticles. Material Science Research India. 15 (3):279-295.
Publisher | Google Scholor - Ren, G. Hu, D. Cheng, E. W. Vargas-Reus, M. A. Reip. et.al. (2009). Characterization of copper oxide nanoparticles for antimicrobial applications. International journal of antimicrobial agents. 33(6):587-590.
Publisher | Google Scholor - Singh, J. Kaur, G. Rawat, M. (2016). A brief review on synthesis and characterization of copper oxide nanoparticles and its applications. J. Bio electron. Nano technol.
Publisher | Google Scholor - Dörner, L. Cancellieri, C. Rheingans, B. Walter, M. Kägi, et.al. (2019). Cost-effective sol-gel synthesis of porous CuO nanoparticle aggregates with tunable specific surface area. Scientific reports. 9(1):1-13.
Publisher | Google Scholor - Sahooli, M. Sabbaghi, S. Saboori, R. (2012). Synthesis and characterization of mono sized CuO nanoparticles. Materials Letters. 81:169-172.
Publisher | Google Scholor - Sahai, A. Goswami, N. Kaushik, S. Tripathi, S. (2016). Cu/Cu2O/CuO nanoparticles: Novel synthesis by exploding wire technique and extensive characterization. Applied Surface Science. 390:974-983.
Publisher | Google Scholor - Nabila, M. I. Kannabiran, K. Biosynthesis, (2018). characterization and antibacterial activity of copper oxide nanoparticles (CuO NPs) from actinomycetes. Bio catalysis and agricultural biotechnology. 15:56-62.
Publisher | Google Scholor - Mohammed, W. M. Mubark, T. H. Al-Haddad, R. M. (2018). Effect of CuO nanoparticles on antimicrobial activity prepared by sol-gel method. Int. J. Appl. Eng. Res. Dev. 13:10559-10562.
Publisher | Google Scholor - Dhineshbabu, N. Rajendran, V. Nithyavathy, N. Vetumperumal, R. (2016). Study of structural and optical properties of cupric oxide nanoparticles. Applied Nanoscience 6:933-939.
Publisher | Google Scholor - Manimaran, R. Palaniradja, K. Alagumurthi, N. Sendhilnathan, S. Hussain, J. (2014). Preparation and characterization of copper oxide nanofluid for heat transfer applications. Applied Nanoscience. 4:163-167.
Publisher | Google Scholor - Zedan, A. F. Mohamed, A. T. El-Shall, M. S. AlQaradawi, S. Y. AlJaber, A. S. (2019). Tailoring the reducibility and catalytic activity of CuO nanoparticles for low temperature CO oxidation. RSC advances. 8(35):19499-19511.
Publisher | Google Scholor - Siddique, S. Waseem, M. Naseem, T. Bibi, A. (2021). Hafeez, et.al. S. Photo-Catalytic and Anti-microbial Activities of r GO/CuO Nanocomposite. Journal of Inorganic and Organometallic Polymers and Materials. 31:1359-1372.
Publisher | Google Scholor - Zhan, Y. Bai, J. Guo, F. Zhou, H. Shu, R. Yu, Y. Qian, L. (2021). Facile synthesis of biomass-derived porous carbons incorporated with CuO nanoparticles as promising electrode materials for high-performance supercapacitor applications. Journal of Alloys and Compounds. 885:161014.
Publisher | Google Scholor - Beigzadeh, M. Pourfayaz, F. Ahmadi, M. H. (2020). Modeling and improvement of solid oxide fuel cell-single effect absorption chiller hybrid system by using nanofluids as heat transporters. Applied Thermal Engineering. 166:114707.
Publisher | Google Scholor - Coelho, L. L. Scaratti, G. Hissanaga, A. M. Oechsler, B. F. José, et.al. (2021). Modeling and fouling control in a hybrid membrane process using CuO-catalytic membrane coupled to ozone. Journal of Environmental Chemical Engineering. 9(5):106138.
Publisher | Google Scholor - Padil, V. V. T. Černík, M. (2013). Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. International journal of nanomedicine. 889-898.
Publisher | Google Scholor - Ahamed, M. Alhadlaq, H. A. Khan, M. M. Karuppiah, P. Al-Dhabi, N. A. Synthesis, (2014). characterization, and antimicrobial activity of copper oxide nanoparticles. Journal of Nanomaterials. 17-17.
Publisher | Google Scholor - Fouda, A. Hassan, S. E.-D. Eid, A. M. Awad, M. A. Althumayri, et.al. (2022). Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity. Green Processing and Synthesis. 11(1):931-950.
Publisher | Google Scholor - Chaerun, S. K. Prabowo, B. A. Winarko, R. (2022). Bio nano technology the formation of copper nanoparticles assisted by biological agents and their applications as antimicrobial and antiviral agents. Environmental Nanotechnology, Monitoring & Management. 18:100703.
Publisher | Google Scholor - Hang, X. Peng, H. Song, H. Qi, Z. Miao, X. Xu, W. (2015). Antiviral activity of cuprous oxide nanoparticles against Hepatitis C Virus in vitro. Journal of virological methods. 222:150-157.
Publisher | Google Scholor - Jayaseelan, C. Abdulhaq, A. Ragavendran, C. Mohan, S. (2022). Phytoconstituents Assisted Biofabrication of Copper Oxide Nanoparticles and Their Antiplasmodial, and Antilarval Efficacy: A Novel Approach for the Control of Parasites. Molecules 27(23):8269.
Publisher | Google Scholor - Meghana, S. Kabra, P. Chakraborty, S. Padmavathy, N. (2015). Understanding the pathway of antibacterial activity of copper oxide nanoparticles. RSC advances. 5(16):12293-12299.
Publisher | Google Scholor - Khatami, M. Heli, H. Mohammadzadeh Jahani, P. Azizi, H. Lima Nobre, M. A. (2017). Copper/copper oxide nanoparticles synthesis using Stachys lavandulifolia and its antibacterial activity. Iet Nanobiotechnology.11(6)709-713.
Publisher | Google Scholor