Research Article
Study of the Change in Physico-Chemical Properties of Acidity Affected Soil with the Application of Different Lime Rates: Incubation Study
- Yohannse Habteyesus Yitagesu *
Ethiopia Institute of Agricultural Research, Holetta Research Center, Ethiopia.
*Corresponding Author: Yohannse Habteyesus Yitagesu, Ethiopia Institute of Agricultural Research, Holetta Research Center, Ethiopia.
Citation: Yohannse H Yitagesu. (2024). Study of the Change in Physico-Chemical Properties of Acidity Affected Soil with the Application of Different Lime Rates: Incubation Study, Clinical and Laboratory Research, BioRes Scientia Publishers. 2(1):1-6. DOI: 10.59657/2994-6441.brs.24.004
Copyright: © 2024 Yohannse Habteyesus Yitagesu, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: September 18, 2023 | Accepted: October 10, 2023 | Published: January 03, 2024
Abstract
Acidic soils are not responsive to the application of inorganic fertilizers without amendments and reclamation by agricultural lime. Liming is the best technology and a conditioner to rises the pH and available phosphorus levels of acid soils. For this research activity, calcite lime in different rates incubated acid soil aims to show the change in physicochemical properties. The soil sample for incubation work has been collected from fifteen locations of acid-prone areas, all over the country. The incubation study has been conducted with four lime rate factors (0, 0.5, 1.0, 1.5, and 2.0*LR) and the rate of CaCO3 has been calculated from the initial soil acidity level by using exchangeable acidity lime recommendation methods. After 90 days of incubation, soil physicochemical parameters have been characterized for pH, Exchangeable acidity, Total nitrogen, Organic carbon, and Available phosphorus. From the study findings, the level of acidity has declined up to 88.96%. The pH level has been improved from 4.98 to 5.61, and the available phosphorus also accordingly improved from 7.69ppm to 8.63ppm but not significant with 0.5-factor intervals. There is no significant response observed in Total Nitrogen and Organic carbon parameters with the application of different lime ratings. From this study conclude that as the rate of lime applied is amended the level of acidity, pH, and phosphorus level also accordingly improved but no significant change was observed for Total nitrogen and Organic carbon. To increase phosphorus content, sufficient and responsible for better amendment of the soil and crop productivity, needs to apply different sources of phosphorus fertilizers. The exchangeable acidity lime recommendation method even with doubled LR factor, might not raise the pH to the optimum level of crop requirement.
Keywords: incubation; soil properties; lime
Introduction
Soil acidity limits or reduces crop production primarily by impairing root growth thereby reducing nutrient and water uptake (Marschner, 2011). It converts available forms of soil nutrients into unavailable forms resulting in poor basic cations and some micronutrients which are essential to crop growth and development (Tisdale et al., 1993; Wang et al., 2006).Soil acidity covers 43% of the total land of Ethiopia and now it’s a critical issue in most highlands of the country because of its impact on soil health and crop productivity (Tessema et al., 2012; Melese and YliHalla, 2016). Acidity associated with Aluminum, Hydrogen, iron, and manganese toxicities to plant roots in the soil solutions and corresponding deficiencies of the available Phosphorus, molybdenum, calcium, manganese, and potassium (Kisinyo et al., 2014). Low nutrient status (e.g., P deficiency) and high concentration of certain elements (e.g., Al, Mn) are two of the most important factors limiting crop production on highly weathered, acid soils. The deficiency of phosphorus occurs through adsorption reactions making it one of the most limiting nutrients for food production in acid soil (Jubrin et al., 2000). The low P status of highly weathered acid soils is a particular problem and large amounts of P need to be applied to raise concentrations of available soil P to an adequate level (Sanchez and Uehara, 1980).
Crop management practices, removal of organic matter, continuous application of acid-forming fertilizers, and microbial production of nitric and sulfuric acids can also contribute to soil acidity (Behera and Shukla, 2015; Fageria and Nascente, 2014). Among the Several practices, cultivation of acid-tolerant plants, covering the surface with non-acidic soil, the use of organic fertilizers, and liming have been recommended to reclaim soil acidity and improve the productivity of acidic soils. Of these practices, the application of agricultural lime and organic fertilizers is considered being the best measure (Chen et al., 2001). Liming is a major and effective practice to overcome soil acidity constraints and improve crop production on acid soils. The important acidity indices (Soil pH, base saturation, and Al saturation) were used as a basis for the determination of lime rates. Besides, crop responses to lime are key tools for making liming recommendations for crops grown on acid soils (Fageria and Baligar, 2008; Rengel, 2011). Lime improves base saturation and availability of calcium and magnesium. Phosphorus and molybdenum fixation is reduced by inactivating the reactive constituents. This research activity therefore aimed on investigating the influence of different lime ratings on the change of physico-chemical properties.
Materials and Methods
Description of the study area
The soil sample was collected from the fourteen acidity affected areas all over the country from Amhara (Adis kdam, Tilili jibayta, banja, and D/Markos), SNNP (Emdibr, Ocholo-Arbaminch, Chencha-Arbaminch, and Bula), Oromiya (Nedjo, Jeldu, Jima, Midakegni and Bedi) and Benishangul Gumuz regions(pawa); Ethiopia. That acidity-affected area was selected based on the quick survey results.
Figure1: Location map of the study area.
Sample Collections and Analysis
About 15kg of composited soil samples at a depth of 20cm were collected from each site for incubation and further laboratory study. The soil samples were air dried, ground, and sieved to pass through a 2.0 mm sieve for physicochemical analysis and soil-lime incubation experiment. For total nitrogen and organic carbon determination, the soil was further pulverized and passed through a 0.5 mm sieve. The initial soil samples were analyzed for pH, Exchangeable cations, Exchangeable acidity, organic carbons, Total nitrogen, and Available Phosphorus.
Incubations
Five hundred grams of soil with four lime rates (0.5*EA, 1.0*EA, 1.5*EA, and 2.0*EA) including no liming (control) was incubated in a greenhouse for three months. Each of the lime rates was replicated two times. The frequency and the amount of water poured during the incubation period are based on the field capacity and friability of the soil. The sample after incubation was completed; it was air-dried, grounded, and sieved by passing through 2.0mm and 0.5mm sieve.
Physico-Chemical Analysis
The initial and incubated soil samples were analyzed for Soil pH in water (1soil:2.5water), Exchangeable cations (Ca, Mg, Na, and K), Exchangeable acidity, Organic carbons (Walkley and Black), Total nitrogen (Kjeldehal) and Available Phosphorus (Bray II).
pH
The pH of the soil sample was measured potentiometrically in the suspension of 1:2.5 soil liquid mixtures. 20 g soil samples were weighed and poured 50 mL of distilled water. The mixture was allowed to stand for 30 min and measured by inserting electrode. Reading is then taken (after 30 to 60 s) to the nearest 0.1 pH unit.
Available phosphorus
The phosphorus content of soil sample was analyzed followed by Bray II method. The insoluble form of Alumunium phosphate and iron phosphate are the major phosphate sources. Acidic ammonium fluoride removes some phosphates ions from insoluble phosphate.
Total Nitrogen
The micro-kjeldahl procedure was followed. The samples were digested in concentrated sulphuric acid with selenium as a catalyst. The solution was then made alkaline with 40% sodium hydroxide; the distillate (evolved ammonia) was trapped in 2% boric acid and titrated with standard acid with 0.01 M HCl (Reeuwijk, LP van ,2002).
Organic carbon
The Walkley-Black procedure was followed. Wet combustion of the organic matter involved with a mixture of potassium dichromate and sulphuric acid. The residual dichromate was titrated against ferrous sulphate and reported as g/kg (Reeuwijk, LP van ,2002).
Results and Discussion
Table 1: Physico-chemical characteristics of the soil.
| Lime rate@ Exchangeable acidity | ||||||
| Parameter | LR | 0 | 0.5 | 1 | 1.5 | 2 |
| PH (1:2.5 H2O) | Min. | 4.59 | 4.79 | 4.93 | 5.09 | 5.21 |
| Max. | 5.74 | 5.83 | 5.85 | 5.88 | 6.21 | |
| Avg. | 4.98 | 5.11 | 5.25 | 5.44 | 5.61 | |
| EX. Acidity (1M KCl) | Min. | 0.43 | 0.3 | 0.25 | 0.16 | 0.09 |
| Max. | 5.84 | 3.42 | 1.38 | 0.9 | 0.7 | |
| Avg. | 2.45 | 1.77 | 0.89 | 0.47 | 0.27 | |
| AV. Phosphorus (Brey II) | Min. | 2.4 | 2.8 | 3.2 | 2.8 | 3.2 |
| Max. | 25.98 | 28.57 | 29.57 | 31.98 | 31.97 | |
| Avg. | 7.69 | 8.36 | 8.37 | 8.52 | 8.63 | |
| %TN (kjeldhal) | Min. | 0.162 | 0.169 | 0.165 | 0.165 | 0.163 |
| Max. | 0.427 | 0.426 | 0.429 | 0.423 | 0.425 | |
| Avg. | 0.262 | 0.262 | 0.263 | 0.261 | 0.261 | |
| %Oc (walkley&black) | Min. | 1.56 | 1.66 | 1.715 | 1.64 | 1.62 |
| Max. | 5.56 | 5.38 | 5.495 | 5.46 | 5.53 | |
| Avg. | 3.31 | 3.41 | 3.442 | 3.59 | 3.46 | |
LR0.00 mean no lime applied, LR0.5: half dose lime applied, LR1.0: full dose lime applied, LR1.5 mean half +full lime dose applied, LR2.0 double dose lime applied
The amount of lime for incubation was obtained from lime rate formula:
Exchangeable acidity and pH
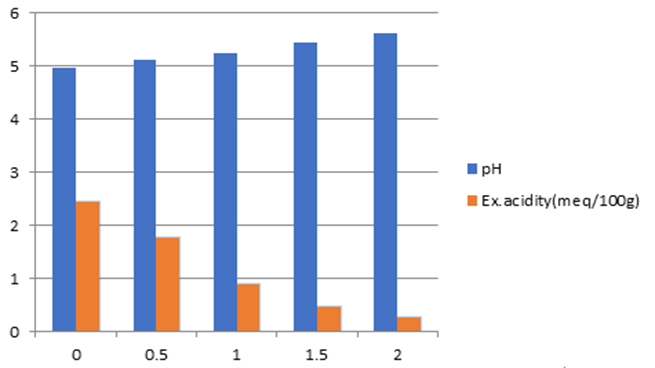
The result has shown that as the rate of lime increased, the level of acidity decreased up to 88.96% with the lime rate factor doubled. With half lime rate increments, between 29.02% to 49.62 Percentage acidity level improvement was observed. At the full dose lime rate, the level of acidity improved from about 64.27% to 71.70% with regard to the initial acidity level. The pH level accordingly improved as the rate of lime increased. It was alleviated from 4.98 to 5.61. However, the optimum pH range between 6.5 to 7.5 cannot be achieved even the rate factor is doubled. From this incubation study with the exchangeable acidity lime rate determination even the rate is doubled cannot get the optimum pH range of 6.5-7.5.
Figure 2: Lime rate factor (x-axis) versus pH & Ex. Acidity (y-axis).
Total nitrogen and Organic carbon
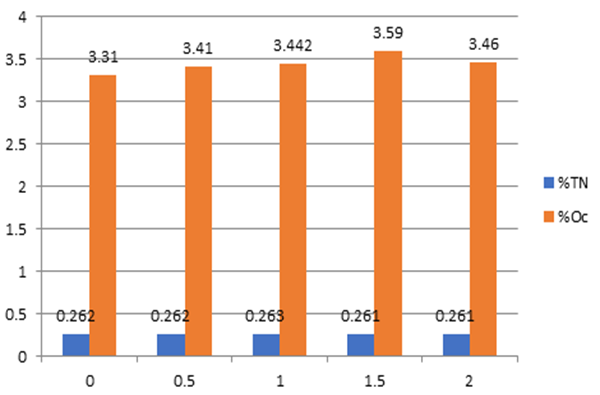
As lime rate increases, there is no significant difference observed in the level of Total nitrogen even the amount of lime is two times the lime requirements. From this finding can conclude that the lime application at different rates doesn’t improve the level of total nitrogen and organic carbon contents of the soil.
Figure 3: Lime rate factor (x-axis) versus TN & Oc (y-axis).
Available phosphorus
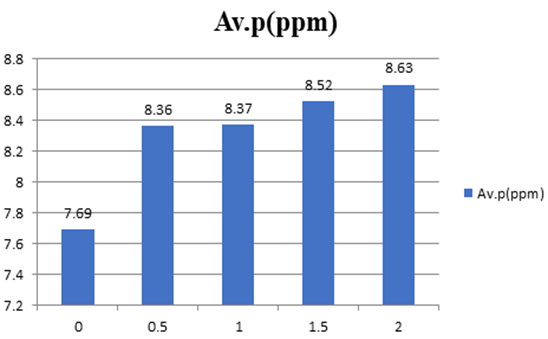
The available phosphorus accordingly improved from 7.69ppm to 8.63ppm. There is a significant difference between ‘no lime treated as compared with lime treated’ but not significant with 0.5-factor intervals of lime treated. From the finding can conclude that even half the lime rate recommended is sufficient to boost phosphorus to be available to plants according to Brey II test.
Figure 4: Lime rate factor (x-axis) versus Av. phosphorus (y-axis).
Conclusions And Recommendations
Liming is a great role to amend soil acidity by improving the pH level and available phosphorus. The carbonate from CaCO3 component reacts with hydrogen ions in the soil solution and raises the soil pH. The main effects of liming are increasing the available P through inactivation or precipitation of exchangeable and soluble Al and Fe hydroxides, and pH of the soil. Even though the available phosphorus level in soil raises by the amendment of lime technology but not sufficient to increase the phosphorus content to the optimum amount. To optimize phosphorus content which is sufficient and responsive for better amendment of the soil and crop productivity needs to apply different sources of phosphorus fertilizers. With the exchangeable acidity lime rate recommendation, the total nitrogen and organic carbon content of the soil doesn’t improve by lime amendment even if the rate is doubled. Lime therefore is a conditioner of acid-affected soil by freeing the fixed phosphors from Aluminum toxicity and raising the pH level. In order to effective the different forms of fertilizers, conditioning the acid soil with lime is critical and its proven in amending acidity.
References
- Behera, S. K., and Shukla, A. K. (2015). Spatial distribution of surface soil acidity, electrical conductivity, soil organic carbon content and exchangeable potassium, calcium and magnesium in some cropped acid soils of India. Land Degrad. Dev, 26:71-79.
Publisher | Google Scholor - Bray R.h., and Kurz L.T. (1945) determination of total, organic, and available forms of phosphorus in soil. Soil Sci, 59:39-45.
Publisher | Google Scholor - Ankerman, D, Large R. (SD) Agronomi hand book, soil and plant analysis, A & L. Agricultural laboratories. Memphis, USA.
Publisher | Google Scholor - Chen, J.H., Wu, J.T., Huang, W.T. (2001). Effects of compost on the availability of nitrogen and phosphorus in strongly acidic soils. Food and Fertilizer Technology Center Extension Bulletin, 155.
Publisher | Google Scholor - Fageria, N., and Baligar, V. (2008). Ameliorating soil acidity of tropical Oxisols by liming for sustainable crop production. Adv. Agron, 99:345-399.
Publisher | Google Scholor - Fageria, N. K., and Nascente, A. S. (2014). Management of soil acidity of South American soils for sustainable crop production. Adv. Agron, 128:221-275.
Publisher | Google Scholor - Kisinyo, P.O., Opala, P.A. Gudu, S.O. Othieno, C.O. Okalebo, J.R. Palapala, V., Otinga, A.N. (2014). Recent advances towards understanding Kenyan acid soils for improved crop production. African Journal of Agricultural Research, 9(31):2397-2408.
Publisher | Google Scholor - Marschner, H. (2011). “Marschners mineral nutrition of higher plants,” Academic press, London.
Publisher | Google Scholor - Melese, A., Yli-Halla, M. (2016). Effects of applications of lime, wood ash, manure and mineral P fertilizer on the inorganic P fractions and other selected soil chemical properties on acid soil of Farta District, northwestern highland of Ethiopia. African Journal of Agricultural Research, 11(2):87-99.
Publisher | Google Scholor - Reeuwijk, LP van. (2002). Technical paper in procedures in soil analysis. 6th edition.
Publisher | Google Scholor - Rengel, Z. (2011). Soil pH, soil health and climate change. In “Soil health and climate change”. Springer, 69-85.
Publisher | Google Scholor - Sanchez, P. A., and Uehara, G. (1980). Management considerations for acid soils with high phosphorus fixation capacity. The role of phosphorus in agriculture, 471-514.
Publisher | Google Scholor - Tessema, G., Argaw, M., Adgo, E. (2012). Farmers soil management practices and their Perceptions to soil acidity at Ankesha District of Awi Zone, Northwestern Ethiopia. Libyan Agriculture Research Center Journal International, 3:64-72.
Publisher | Google Scholor - Tisdale, S., Nelson, W., Beaton, J., and Havlin J. (1993). Soil acidity and basicity. Soil Fertility and Fertilizers, 5th ed. Macmillan, New York, 364-404.
Publisher | Google Scholor - Wang, J., Raman, H., Zhang, G., Mendham, N., and Zhou, M. (2006). Aluminium tolerance in barley (Hordeum vulgare L.): physiological mechanisms, genetics and screening methods. J. Zhejiang University Sci, 7:769-787.
Publisher | Google Scholor