Research Article
Study of Correlation of Dyslipidaemia and Diabetes Mellitus with Hypothyroidism in Middle Aged Punjabi Females & Its Impact on Non-Alcoholic Fatty Liver Disease
1. Department of Medicine, Guru Nanak Dev Hospital, Amritsar attached to Government Medical College, Amritsar, India.
2. Scientific Director & Owner of Dr Kulvinder Kaur Centre for Human Reproduction, Jalandhar, Punjab, India.
*Corresponding Author: Kulvinder Kochar Kaur, Scientific Director & Owner of Dr Kulvinder Kaur Centre for Human Reproduction, Jalandhar, Punjab, India.
Citation: K. Ishrat, Kulvinder K. Kaur. (2024). Study of Correlation of Dyslipidaemia and Diabetes Mellitus with Hypothyroidism in Middle Aged Punjabi Females & Its Impact on Non-Alcoholic Fatty Liver Disease, International Clinical and Medical Case Reports, BioRes Scientia Publishers. 3(2):1-10. DOI: 10.59657/2837-5998.brs.24.031
Copyright: © 2024 Kulvinder Kochar Kaur, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: December 19, 2023 | Accepted: January 08, 2024 | Published: January 30, 2024
Abstract
Introduction: Thyroid hormones (THs) play critical roles in growth, differentiation and metabolism. Since hypothyroidism is encountered in females more than in males and the idiopathic form of hypothyroidism occur mainly in middle aged females so we conducted a study of correlation of dyslipidemia and diabetes mellitus with hypothyroidism in middle aged Punjabi females.
Materials and methods: The present observational study was conducted in the department of Medicine, Guru Nanak Dev Hospital, Amritsar attached to Government Medical College, Amritsar. The study was conducted after approval from Institutional Ethical Committee. Female patients in age group of 30 to 60 years who were diagnosed with clinical or subclinical hypothyroidism were included in the study and evaluated for dyslipidemia and Diabetes Mellitus.
Results: The incidence of increased total cholesterol, triglyceride, LDL and VLDL was 89%, 88%, 80% and 95% respectively. The incidence of reduced HDL was 72%. The incidence of increased FBS, Postprandial glucose and HbA1c was 68%, 78% and 87% respectively. A significant positive correlation was found between thyroid hormone and total cholesterol, triglyceride, LDL and VLDL. A significant negative correlation was found between thyroid hormone and HDL. A significant positive correlation was found in between thyroid hormone and FBS, RBS and HbA1c. A significant positive correlation was found between HbA1c and total cholesterol, triglyceride, LDL and VLDL. A significant negative correlation was found between HbA1c and HDL.
Conclusion: The relationship between hypothyroidism and lipid profile as well as diabetes mellitus is characterized by a complex interdependent interaction. To conclude there is a high prevalence of dyslipidemia and diabetes mellitus in hypothyroid patients. Further extrapolation in non-alcoholic fatty-liver disease (NAFLD)has been detailed.
Keywords: diabetes mellitus; dyslipidemia; females; hypothyroidism
Introduction
Introduction
Thyroid hormones (THs) play critical roles in growth, differentiation and metabolism. The two most important thyroid hormones are thyroxine (T4) and triiodothyronine (T3). Thyroid stimulating hormone (TSH), which is produced by pituitary gland, acts to stimulate hormone production by thyroid gland. Pituitary gland is stimulated to make TSH by hypothalamus gland [1]. The metabolic manifestations of the thyroid disease related to either excessive or inadequate production of thyroid hormones (hyperthyroidism and hypothyroidism, respectively) are known as thyroid disorders [2]. Hypothyroidism is associated with many biochemical abnormalities like dyslipidemia [3]. Levels of total cholesterol and low-density lipoprotein cholesterol increase as thyroid function declines [4]. Thus, hypothyroidism is one of the important causes of secondary dyslipidemia [5].
The prevalence of hypothyroidism is 1.4–13% in patients with hyperlipidemia, indicating that thyroid failure is common and may often go undetected in these patients [6]. Thyroid diseases and diabetes mellitus are the two most common endocrine disorders. The prevalence of thyroid diseases is higher in people with diabetes mellitus than those without diabetes mellitus. Abnormality in one hormone level may alter the functional state of other hormone as diabetes and thyroid disorders have influence on each other and associations between both conditions have long been reported. Thyroid hormones contribute to the regulation of carbohydrate metabolism and pancreatic function. According to WHO, there were 31.7 million persons with diabetes in India in 2000 and this number is likely to be increased up to 71.4 million in 2030 [7,8]. Since hypothyroidism is encountered in females more than in males and the idiopathic form of hypothyroidism occur mainly in middle aged females so we conducted a study of correlation of dyslipidemia and diabetes mellitus with hypothyroidism in middle aged Punjabi females.
Material and Method
The present study entitled “Study of correlation of dyslipidemia and diabetes mellitus with hypothyroidism in middle aged Punjabi females” was conducted in the department of Medicine, Guru Nanak Dev Hospital, Amritsar attached to Government Medical College, Amritsar. This was an observational study in which patients who presented to Medicine department of Guru Nanak Dev Hospital and fulfilled the inclusion criteria of the study were evaluated for dyslipidemia and Diabetes Mellitus.
The study was conducted after approval from Institutional Ethical Committee. The patients were explained in their vernacular language about the procedures to be adopted in the study and their informed consent was taken and Performa of the profile of the patient was filled up.
Inclusion criteria
Female patients in age group of 30 to 60 years who were diagnosed with clinical or subclinical hypothyroidism.
Exclusion criteria
Patients on any hypolipidemic drug in past 3 months.
Alcoholic patients.
Critically ill patients.
Patient on OCP and other lipid modifying drugs.
Male patients.
Females below 30 years of age and above 60 years of age.
Patients who were known cases of diabetes mellitus.
Patients of known dyslipidemia.
Pregnant women.
Method of Study
All the patients who fulfilled the inclusion criteria were included in the study. On admission, detailed history and clinical examination of the patients were done. Primary hypothyroidism was diagnosed by a high serum thyroid-stimulating hormone (TSH) concentration and a low serum free thyroxine (T4) concentration, whereas subclinical hypothyroidism was diagnosed by a normal free T4 concentration in the presence of an elevated TSH concentration. Secondary (central) hypothyroidism was diagnosed by a low serum T4 concentration and a serum TSH concentration that was not appropriately elevated. Subclinical hypothyroidism was diagnosed as a serum thyroid-stimulating hormone (TSH) level above the upper limit of normal despite normal levels of serum free thyroxine. In this study, routine baseline investigations were performed i.e., Hb, TLC, DLC, PBF, Blood urea, Serum creatinine, Serum bilirubin, SGOT, SGPT, TSP, DSP, urine complete examination, ECG, FBS, RBS (2 hours postprandial RBS), HbA1C, TSH, free T3, free T4 and Lipid profile Diabetes mellitus was labelled according to ADA (American Diabetes Association) guidelines, 2018: Fasting plasma glucose ≥126mg/dL (7.0mmol/L). Fasting was defined as no caloric intake for at least 8 h.
Or 2 hours postprandial glucose ≥200 mg/dL (11.1mmol/L) during OGTT. The test was performed as described by the WHO, using a glucose load containing the equivalent of 75-g anhydrous glucose dissolved in water. Or HbA1C ≥6.5% (48 mmol/mol). The test was performed in a laboratory using a method that is NGSP certified and standardized to the DCCT assay. Or in a patient with classic symptoms of hyperglycemia or hyperglycemic crisis, a random plasma glucose level ≥200mg/dL (11.1 mmol/L).
Freidwald formula
LDL=Total cholesterol – HDL
VLDL=TG/5
DATA ANALYSIS
The data was collected systematically and analysed statistically using pearsons correlation.
Results and observations
Table 1: Prevelance of Dylipidemia and Diabetes Mellitus in Hypothyroid Patients
| Parameter | Number of Patience | Percentage | |
| Total cholesterol | Elevated (>200 mg/dl) | 89 | 89% |
| Triglyceride | Elevated (>150 mg/dl) | 88 | 88% |
| HDL | Reduced (<50> | 72 | 72% |
| LDL | Elevated (>130 mg/dl) | 80 | 80% |
| VLDL | Elevated (>30 mg/dl) | 95 | 95% |
| FBS | Elevated (≥126 mg/dl) | 68 | 68% |
| Postprandial glucose | Elevated (≥200 mg/dl) | 78 | 78% |
| HbA1c | Elevated (≥6.5%) | 87 | 87% |
Table 2: Correlation Between Hypothyroidism and Lipid Profile in Middle Aged Females with Hypothyroidism
| Thyroid hormone | R (pearsons correlation) | P value |
| Total cholesterol | 0.86 | 0.00 |
| Triglyceride | 0.94 | 0.01 |
| HDL | -0.51 | 0.02 |
| LDL | 0.86 | 0.00 |
| VLDL | 0.94 | 0.01 |
Table 3: Correlation between Hypothyroidism and Diabetes Mellitus in middle Aged Females with Hypothyroidism
| Thyroid hormone | R (pearsons correlation) | P value |
| FBS | 0.85 | 0.001 |
| RBS | 0.92 | 0.011 |
| HbA1c | 0.84 | 0.001 |
Table 4: Correlation Between Dyslipidemia and Diabetes Mellitus in Middle Aged Females with Hypothyroidism
| HbA1c | R (pearsons correlation) | P value |
| Total cholesterol | 0.96 | 0.001 |
| Triglyceride | 0.94 | 0.01 |
| HDL | -0.80 | 0.00 |
| LDL | 0.96 | 0.001 |
| VLDL | 0.88 | 0.02 |
Discussion
Amongst 100 patients included in present study 96% were clinical hypothyroid and 4% were subclinical hypothyroid. The mean age of the patients in the present study was 49.36 ±9.37years. Patients were between 30-60 years of age. Most of the patients were from age group 51-60 years followed by 41-50 years and 30-40 years. The mean TSH levels were 16.6 mlU/l. TSH levels were between 6-64 mlU/l. The mean free T3 levels were 0.49 ng/dL ranging in between 0.1- 4.2 ng/dL. The mean free T4 levels were 2.98 µg/dL ranging in between 0.2-9 µg/dL. The mean FBS levels were 157.18 mg/dl ranging in between 70-300 mg/dl. The mean RBS levels were 249.1 mg/dl ranging in between 102-568 mg/dl. Mean HbA1c levels were 7.88% ranging in between 4-11.4%. Mean total cholesterol, triglycerides, HDL, LDL and VLDL levels were 403.41 mg/dl, 247.05 mg/dl, 50.24 mg/dl, 303.02 mg/dl and 71.45 mg/dl respectively. Total cholesterol, triglycerides, HDL, LDL and VLDL levels ranged between 80-1000 mg/dl, 100-580 mg/dl, 20-200 mg/dl, 32-890 mg/dl and 28-290 mg/dl respectively.
On the basis of TSH levels patients were divided into three groups. Group 1, group 2 and group 3 with TSH levels (<10>20 mlU/L). Group 1 consisted of 5 patients with mean TSH levels 7.66 mlU/L, group 2 consisted of 78 patients with mean TSH levels 13.16mlU/L and group 3 consisted of 17 patients with mean TSH levels 35.35mlU/L. On comparison among three groups, the mean cholesterol levels were higher in group 3 (529.0) followed by group 2 (407.29) and group 1 (267.0) but the difference was not statistically significant. The mean triglyceride levels in group 3 (252.28) were higher than group 2 (231.17) followed by group 1 (219.6) but the difference was not statistically significant. Mean HDL levels were higher in group 2 (51.52) followed by group 3 (48.05) and group 1(37.6) but the difference was not statistically significant. Mean LDL levels were higher in group 3 (328.70) followed by group (305.06) and group 1(183.8) but the difference was not statistically significant. Mean VLDL levels were higher in group 3 (84.4) followed by group 2 (70.79) and group 1(70.64) but the difference was not statistically significant. The mean FBS levels were 157.18 mg/dl ranging in between 70-300 mg/dl. The mean RBS levels were 249.1 mg/dl ranging in between 102-568 mg/dl. Mean HbA1c levels were 7.88% ranging in between 4-11.4%. Mean FBS levels were higher in group 3(203.2) followed by group 2(174.58) and group 1(150.43) and the difference was statistically significant. On comparison mean post-prandial blood glucose levels were higher in group 3(321.6) followed by group 2(274.47) and group 1 (238.9) and the difference was statistically significant. On comparison mean HbA1c levels were higher in group 3(8.67) followed by group 2(7.75) and group 1(7.14) but the difference was not statistically significant.
The prevalence of elevated total cholesterol, triglyceride, HDL, LDL, VLDL, FBS, postprandial glucose and HbA1c were 89%, 88%, 72%, 80%, 95%, 68%, 78% and 87% respectively. A positive correlation was found between thyroid hormone and total cholesterol. The P-Value is 0.00. The result is significant at p<.05. A positive correlation was found between thyroid hormone and triglyceride. The P-Value is 0.01. The result is significant at p<.05. A negative correlation was found between thyroid hormone and HDL. The P-Value is 0.02. The result is significant at p<.05. A positive correlation was found between thyroid hormone and LDL. The P-Value is 0.00. The result is significant at p<.05. A positive correlation was found between thyroid hormone and VLDL. The P-Value is 0.01. The result is significant at p<.05. Desai JP et al9 conducted a study to find whether there is any correlation between serum lipid levels and hypothyroidism (subclinical and overt). In patients with subclinical hypothyroidism, they found elevated serum total cholesterol and triglyceride levels as compared to controls but no significant difference was seen between HDL levels. In patients with overt hypothyroidism, elevated serum total cholesterol and triglycerides were found as compared to controls but HDL level was found to be decreased. There are limited studies about the correlation between hypothyroidism and blood glucose levels. Some previous studies found that recurrent hypoglycemic episodes are the presenting signs for the development of hypothyroidism in patients with type 1 diabetes and replacement with thyroid hormones reduced the fluctuations in blood glucose levels [10].
A positive correlation was found between thyroid hormone and FBS. The P-Value is 0.001. The result is significant at p<.05. A positive correlation was found between thyroid hormone and RBS. The P-Value is 0.011. The result is significant at p<.05. A positive correlation was found between thyroid hormone and HbA1c. The P-Value is 0.001. The result is significant at p<.05. Karar T et al [11] in 2015 conducted a study to evaluate the levels of thyroid hormones and glycosylated haemoglobin (HbA1c) among patients and correlated thyroid hormones with HbA1c and different types of lipids among diabetic patients. The result showed increased mean levels of HbA1c (8.4%) and normal level of thyroid stimulating hormone (TSH) (4.5 mlU/L) and T4 (14.1 pmol/L). The results also showed a weak positive correlation between HbA1c and TSH and insignificant correlation with thyroxine. In the present study a positive correlation was found between HbA1c and total cholesterol. The P-Value is 0.001. The result is significant at p<.05. A positive correlation was found between HbA1c and triglyceride. The P-Value is 0.01. The result is significant at p<.05. A negative correlation was found between HbA1c and HDL. The P-Value is 0.00. The result is significant at p<.05. A positive correlation was found between HbA1c and LDL. The P-Value is 0.001. The result is significant at p<.05. A positive correlation was found between HbA1c and VLDL. The P-Value is 0.02. The result is significant at p<.05. Begum A [12] et al in their study found significant correlations between HbA1c value and serum levels of TC, TG and HDL-C but no significant correlation of HbA1c value with LDL-C was found in diabetes patient. Their study concluded that HbA1c value correlated well with lipid profile in diabetes patients.
Further extrapolating our research observations in other obesity associated morbidities like non-alcoholicfatty liver disease (NAFLD) as well as non-alcoholic steatohepatitis (NASH), subsequent to our previous publications on NAFLD as well as NASH etiopathogenesis in addition to their treatment here we further add how hypothyroidism might be impacting NAFLD as well as NASH [13-20].
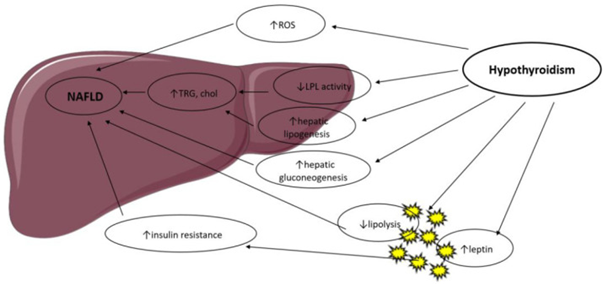
Thyroid hormones (TH) regulate body weight, lipid along with the carbohydrate metabolism as well as thermogenesis. They control lipid metabolism by illustrating particular actions on the liver in addition to adipose tissue (AT), summarized in Figure 1[reviewed in ref no 21], in a coordinated way however with occasionally controversial actions [22]. T3 controls the expression of genes implicated in hepatic lipogenesis along with the genes implicated in the fatty acid oxidation (FFA) via the thyroid hormone receptor-β, which is the major isoform expressed in the liver [23,24]. Thyroid hormone receptor-α is the major modulator of thyroid hormone (TH) effects in the heart as well as brown adipose tissue (BAT). Thus, TH control lipid metabolism in a tissue-based way, along with this was corroborated by studies in knockout (KO) mice. THR-α-knockout mice illustrate diminished liver fat quantities as well as white adipose tissue (WAT)mass via a reduction in genes implicated in lipogenesis. They possess lesser insulin resistance (IR) in addition to hepatic steatosis [24]. THR-β-knockout animals display an escalated liver mass along with the hepatic lipid accrual via escalation of lipogenic genes as well as decreased fatty acid β-oxidation but no significant alteration in WAT [25].
Hyperthyroidism has been illustrated to escalate AT lipolysis [26] as well as hepatic lipogenesis along with is correlated with lower body weight, noticeably in view of escalated catabolism [27]. These actions are modulated by a T3-induced escalation of the expression of variable lipogenic genes (for instance acyl-CoA-synthetase, fatty acid synthase (FAS), acetyl-CoA carboxylase (ACC) along with glucose-6-P dehydrogenase(G6PD)) as well as genes implicated in FFA (for instance lipoprotein lipase (LPL), fatty acid-binding protein (FABP) as well as fatty acid transporter (FAT) [23]. Hypothyroidism decreased liver uptake of FFA obtained from triglycerides [28], in additionto is correlated with a diminished lipolysis in the AT along with diminished cholesterol clearance [29]. Resultantly, β oxidation of FFA as well as triglyceride clearance is decreased, with a concurrent hepatic accrual of triglycerides along with escalation of low-density lipoprotein (LDL) uptake. Hypothyroidism diminishes hepatic lipase activity, which modulates FFA in addition to oxidation of long-chain fatty acids(lCFAs) for energy generation.Lipid storage in the liver is further escalated by obesity along with lesser resting energy expenditure(EE), both escalated by hypothyroidism [28]. TH treatment in human along with murine models reverts hepatic lipase diminished activity.
In the mitochondria, TH stimulate carnitine palmitoyltransferase-1a(Cpt1a), the rate-limiting enzyme in FFA.
Obesity, in both human and animal studies, is found to result in lipid accumulation in the liver, leading to fibrosis in additionto cirrhosis. Escalated hepatic lipid deposition stimulates downregulation of variable metabolism- associated genes, which are dependent of T3 actions [23].Thyroid hormones are activators of lipogenesis through direct in additionto indirect modes .T3 stimulates enzymes that catalyze various significant steps of hepatic fatty acid generation, for instance ACC (which catalyzes the carboxylation of acetyl-CoA to malonyl-CoA, the first step of hepatic fatty acid synthesis) along with FAS [30].T3 also stimulates variable transcription factors that take part in de novo lipogenesis(DNL), for instance carbohydrate responsive element-binding protein (ChREBP), a robust lipogenic controller [31]. Thyroid-stimulating hormone (TSH) is further believed to stimulate hepatic lipogenesis via binding with the TSH-receptor expressed at the surface of the hepatocytes, which further results in stimulation of the peroxisome proliferator-activated receptor-γ (PPARγ) pathway along with activation of sterol regulatory element-binding transcription factor 1 (SREBP-1c) [32,33]. TSH directly increases hepatic gluconeogenesis as well as diminishes phosphorylationof 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the main target of statins, thereby inducing hypercholesterolemia [34]. Animal studies have suggested a role of T3 in hepatic mitochondrial turnover, which is changed in nonalcoholic fatty liver disease (NAFLD).TH apparently enhance mitochondrial biogeneration along with mitophagy of nuclear receptors [35]. Conversely, hepatic steatosis leads to the repression of T3-dependent genes implicated in metabolism in both humans in addition to animal models [36].
Thyroid hormone signaling further reacts to cross-talk amongst thyroid receptors along with other nuclear receptors sensitive to circulating metabolite quantities, for instance PPARs as well as the liver X receptor (LXR) [37]. Changes of lipophagy, the modes of autophagy of lipid droplets, that is a significant step of lipid mobilization in the liver, is further thought to be taking part in NAFLD, as well as T3 has been illustrated to stimulate lipophagyin vitro along with in vivo [38]. Oxidative stress from β-oxidation is believed to aid in the propagation of nonalcoholic steatohepatitis (NASH) to hepatocyte inflammation as well as liver fibrosis. Hyperthyroidism has been illustrated to enhance oxidative stress (OS), resulting in liver cell damage [39], whereas hypothyroidism diminishes OS quantities via a reduction in energy expenditure [40]. Thus, TH might aid in the propagation of NAFLD to NASH, however the precise pathophysiological modes need clarification.
Figure 1: Courtesy ref no-21-Possible mechanisms in the association between hypothyroidism and NAFLD. LPL: Lipoprotein Lipase; ROS: Reactive Oxygen Species; TRG: Triglyceride; Chol: Cholesterol.
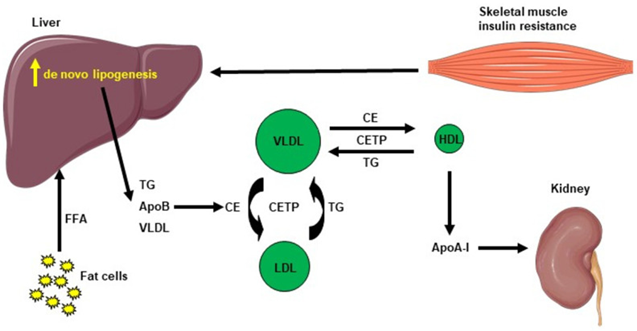
The pathophysiology of NAFLD is, complicated multifactorial in addition to implicates numerous systemic changes [41]. The classical “two-hit” theory is divided into a first “hit” with intrahepatic accrual of fatty acids along with a second “hit” [rev by us in ref no 19], which is inclusive of other factors for instance oxidative stress (OS) as well as mitochondrial impairment. However, this posit has been believed to be inadequate to fully portray the pathogenesis of NAFLD. Thereby, it has been replaced by the “multiple parallel hits” posit that more precisely portray the events of NAFLD generation in addition to propagation. Actually, variable factors, for instance genetic in addition to environmental factors (noticeably dietary habits), work in parallel along with in a synergistic manner to cause NAFLD [42,43]. NAFLD takes place in view of hepatic lipid accrual that will subsequently result in hepatic insulin resistance (IR), changes in gut microbiota (GM) as well as other inimical sequelae for instance mitochondrial impairment, endoplasmic reticulum (ER) stress, OS in addition to generation of reactive oxygen species (ROS) [44]. Following These variable inimical sequelae, a chronic inflammatory state results in the liver, facilitating NAFLD as well as NASH [45]. Hepatic lipid accrual comprises of various lipid intermediates, for instance triglycerides, which are usually believed to be placid, along with diacylglycerols (DAG) in addition to ceramides, that have been illustrated to lead to hepatic IR in variable animal models of NAFLD [45–49]. Furthermore, IR facilitates hepatic de novo lipogenesis (DNL) as well as AT lipolysis, resulting in an escalated flux of free fatty acids (FFAs) to the liver [50]. This event is further correlated with escalated plasma triglycerides (TG) quantities, diminished plasma HDL quantities, aiding in the development of atherogenic dyslipidemia observed in NAFLD [51]. The plasma HDL quantities are generally lesser in insulin-resistant states, which might be reasoned out by the following modes: i) VLDL TG can be exchanged for HDL cholesterol in the of existence of escalated plasma VLDL quantities in addition to the normal activity of cholesteryl ester transfer protein, where a VLDL particle transfers a molecule of TG to an HDL particle in return for one of the cholesteryl ester molecules from HDL. This mode results in a cholesterol-rich VLDL remnant particle which is atherogenic along with a TG enriched, cholesterol- elimination HDL particle [52]. The TG-rich HDL particle will then undergo further alteration, noticeably hydrolysis of its TG, which will result in the dissociation of the apoA-1 protein. Following that, the free apoA-1 would be cleared at a greater pace in the plasma in contrast to the apoA-1 bound to HDL particles, and this event led to diminished circulatingapoA-1, HDL cholesterol in addition to the absolute number of HDL particles [53], as summarized in Figure 2. Overall, these events result in the generation of decontrolled lipid metabolism observed in NAFLD.
Figure 2: Courtesy ref no-21-Cholesterol metabolism induced by hepatic de novo lipogenesis. Skeletal muscle insulin resistance increases hepatic de novo lipogenesis, leading to increased hepatic triglycerides (TG). TG can be exchanged for high-density lipoprotein (HDL) cholesterol in the presence of increased plasma very low–density lipoprotein (VLDL) concentrations and normal activity of cholesteryl ester transfer protein (CETP). A VLDL particle then donates a molecule of TG to an HDL particle in return for one of the cholesteryl esters (CE) molecules from HDL. The TG-rich HDL particle can be hydrolysed of its TG, leading to dissociation of the Apolipoprotein A-1 (Apo A-1) protein. The resulting free Apo A-1 is cleared more rapidly in plasma than the apo A-1 bound to HDL particles, leading to reduced circulating apo A-1, HDL cholesterol and the number of HDL particles.
Conclusion
The relationship between hypothyroidism and lipid profile as well as diabetes mellitus is characterized by a complex interdependent interaction. Unidentified dyslipidemia and diabetes mellitus could negatively impact thyroid function. Therefore, there is a need for routine assay of lipid profile and blood glucose levels in hypothyroid patients to improve the medical management as well as to reduce the morbidity in them. To conclude there is a high prevalence of dyslipidemia and diabetes mellitus in hypothyroid patients Thus it is advocated to routinely monitor the glucose quantities. as well as lipid profile in patients having hypothyroidism. This hypothyroidism- correlated dyslipidemia gets followed by intrahepatic accrual of fat, resulting in non-alcoholic fatty liver disease (NAFLD), that causes the generation of hepatic insulin resistance (IR). The prevalence of NAFLD in the western world is escalating, in addition to that there is accrual of its correlation with hypothyroidism. In view of hypothyroidism has been isolated in the form of a modifiable risk factor of NAFLD along with the recent outcomes obtained corroborate that selective thyroid hormone receptor β (THR-β) agonists are efficacious in the treatment of dyslipidemia as well as NAFLD, interest in plausible therapeutic options for NAFLD targeting these receptors is escalating. Thus, we have provided how the acknowledgement of this correlation of hypothyroidism with regard to dyslipidemia as well as metabolic impairment might be applicable in avoidance of NAFLD progression to NASH as well.
References
- Nussey S, Whitehead S. (2001). Endocrinology: An Integrated Approach. Oxford: BIOS Scientific Publishers. Chapter 3: The thyroid gland.
Publisher | Google Scholor - Jawad AH, Alsayed R, Ibrahim AE, Hallab Z, et al. (2016). Research Journal of Pharmaceutical, Biological and Chemical Sciences. 7(6):1336-43.
Publisher | Google Scholor - Greenspan FS. (2004). The thyroid gland. In: Greenspan FS & Gardner DG (eds). Basic & Clinical Endocrinology. 7th edn. New York: The McGraw-Hill Companies. 215-294.
Publisher | Google Scholor - Liberopoulos EN, Elisaf MS. (2002). Dyslipidemia in patients with thyroid disorders.
Publisher | Google Scholor - Shin DJ, Osborne TF. (2003). Thyroid hormone regulation and cholesterol metabolism are connected through Sterol Regulatory Element- Binding Protein-2 (SREBP-2). J Biol Chem 278:34114-8.
Publisher | Google Scholor - Kuusi T, Saarinen P, Nikkila EA. (1980). Evidence for the role of hepatic endothelial lipase in the metabolism of plasma high density lipoprotein2 in man. Atherosclerosis 36: 589-93.
Publisher | Google Scholor - (2002). Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. Jama. 288(3):321-33.
Publisher | Google Scholor - Kronenberg F. (1990). Hot Flashes: Epidemiology and Physiology. Ann NY Acad Sci. 592:52-86.
Publisher | Google Scholor - Desai JP, Vachhani UN, Modi G, Chauhan K. (2015). A study of correlation of serum lipid profile in patients with hypothyroidism. International Journal of Medical Science and Public Health. 4(8):1108-12.
Publisher | Google Scholor - Leong M, Wilding WJ and MacFarlane I. (1999). Clinical presentation of thyroid dysfunction and Addison’s disease in young adults with type 1 diabetes. Postgraduate Medical. 75:467-70.
Publisher | Google Scholor - Karar T, Alhammad RS, Fattah MA, Alanazi A, Qureshi S. (2015). Relation between glycosylated hemoglobin and lipid and thyroid hormone among patients with type 2 diabetes mellitus at King Abdulaziz Medical City, Riyadh. J Nat Sc Biol Med. 6:75-9.
Publisher | Google Scholor - Begum A, Irfan SR, Hoque MR, Habib SH, Parvin S, et al. (2019). Relationship between HbA1c and Lipid Profile Seen in Bangladeshi Type 2 Diabetes Mellitus Patients Attending BIRDEM Hospital: A Cross-Sectional Study. Mymensingh Med J. 28(1).
Publisher | Google Scholor - Kulvinder Kochar Kaur, Allahbadia GN, Singh M. (2020). How do we apply advances in knowledge of Hepatic Macrophages in treating Liver Diseases especially non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepapititis (NASH), with the increasing incidence of Diabesity-A Systematic Review.EC Endocrinology and Metabolic Research.
Publisher | Google Scholor - Kulvinder Kochar Kaur, Allahbadia GN, Singh M. (2020). An Update on Further Progression of NAFLD, NASH with Prospective Therapies Like L-Carnitine (LC), Nicotinamide Ribose (NR) Combination, as well as Apical Sodium Dependent Bile Acids Transporter (ASBT) or Volixibat and Silybin as Alternatives. Int J Clin Med Cases. 3(3):138.
Publisher | Google Scholor - Kulvinder Kochar Kaur, Allahbadia GN, Singh M. (2021) Mechanisms that associate extension of Nonalcoholic fatty liver diseases (NAFLD) to NASH (Nonalcoholic steatohepatitis) and further progressing to cirrhosis and Hepatocellular carcinoma(HCC) in addition to few proposed biomarkers for poor prognosis.J Endocrinol.1(16):1-18.
Publisher | Google Scholor - Kulvinder Kochar Kaur, Allahbadia GN, Singh M. (2021). How can we optimize therapy of Non-Alcoholic Fatty Acid Liver Disease-A Short Communication on role of Astragaloside IV and other prospective agents”. Clinical Research and Clinical Case Reports. 1(3):1-4.
Publisher | Google Scholor - Kulvinder Kochar Kaur, Allahbadia GN, Singh M. (2021). An update on management of Nonalcoholic Fatty Liver Disease &Nonalcoholic Steatohepapititis-Is the time ripe for achieving resolution of NAFLD &NASH soon. J Endocrinol Res. 3(2):44-60.
Publisher | Google Scholor - Kulvinder Kochar Kaur, Allahbadia GN, Singh M. (2021). The Association of Non-Viral Liver Diseases from NAFLD to NASH to HCC with the Pandemic of Obesity, Type 2 Diabetes,or Diabesity & Metabolic Syndrome –Etiopathogenetic Correlation along with Utilization for Diagnostic &Therapeutic Purposes-A Systematic review’’. J Endocrinol Res. 3(2):10-34.
Publisher | Google Scholor - Kulvinder Kochar Kaur, Allahbadia GN, Singh M. (2022). An update on the Association of Gut-Liver Axis with Gut Microbiome Dysbiosis Correlated NAFLD along with NAFLD- HCC with Potential Therapeutic Approaches: a systematic review. J Hepatol Gastroenterol.
Publisher | Google Scholor - Kulvinder Kochar Kaur, Allahbadia GN, Singh M. (2023). An update on the etiopathogenesis of NAFLD including Organokines for early diagnosis and improvement of management and preventing early HCC development: A narrative review. Liver Res Open J. 4(1): 18-41.
Publisher | Google Scholor - MavromatiM, Jornavyaz FR. (2021). Hypothyroidism- associated dyslipidemia: potential molecularmechanisms leading to NAFLD. Int J Mol Sci 22:12797.
Publisher | Google Scholor - Sinha RA, You SHZhou J, SiddiqueMM, Bay BH, Zhu Xet al. (2012). Thyroid hormone stimulates hepatic lipid catabolism via activation of autophagy. J. Clin. Investig. 122, 2428–2438.
Publisher | Google Scholor - HuangYY, Gusdon AM, Qu S. (2013). Cross-talk between the thyroid and liver: A new target for nonalcoholic fatty liver disease treatment. World J Gastroenterol. 1: 8238–8246.
Publisher | Google Scholor - Flores-Morales A, Gullberg H, Fernandez L, Stahlberg N, LeeNH, VennstromB, Norstedt G. (2002). Patterns of liver gene expression governed by TRbeta. Mol Endocrinol 16: 1257–1268.
Publisher | Google Scholor - Jornayvaz FR, LeeHY, Jurczak MJ, Alves TC, et al. (2012). Thyroid hormone receptor-alpha gene knockout mice are protected from diet-induced hepatic insulin resistance. Endocrinology. 153, 583–591.
Publisher | Google Scholor - Riis AL, Gravholt CH, Djurhuus CB, Norrelund Jorgensen JO, Weeke J, et al. (2002). Elevated regional lipolysis inhyperthyroidism. J Clin Endocrinol Metab 87:4747–4753.
Publisher | Google Scholor - Cachefo A, Boucher P, Vidon C, Dusserre E, Diraison F, er al. (2001). Hepatic lipogenesis and cholesterol synthesis in hyperthyroid patients. J Clin Endocrinol Metab. 86; 5353–5357.
Publisher | Google Scholor - Klieverik LP, Coomans CP, Endert E, Sauerwein HP, Havekes LM, et al. Thyroid hormone effects on whole-body energy homeostasis and tissue-specific fatty acid uptake in vivo. Endocrinology. 150: 5639–5648.
Publisher | Google Scholor - Nedvidkova J, Haluzik M, Bartak V, Dostalova I, Vlcek P, et al. (2004). Changes of noradrenergic activity and lipolysis in the subcutaneous abdominal adipose tissue of hypo- and hyperthyroid patients:An in vivo microdialysis study. Ann N Y Acad Sci. 101: 541–549.
Publisher | Google Scholor - D’Ambrosio R, Campi I, Maggioni M, Perbellini R, et al. (2021). The relationship between liver histology and thyroid function tests in patients with non-alcoholic fatty liverdisease (NAFLD). PLoS ONE. 16: e0249614.
Publisher | Google Scholor - Yin L, Zhang Y, Hillgartner FB. (2002). Sterol regulatory element-binding protein-1 interacts with the nuclear thyroid hormonereceptor to enhance acetyl-CoA carboxylase-alpha transcription in hepatocytes. J Biol Chem 277: 19554–19565.
Publisher | Google Scholor - Dentin R, Girard J, Postic C. (2005). Carbohydrate responsive element binding protein (ChREBP) and sterol regulatory elementbinding protein-1c (SREBP-1c): Two key regulators of glucose metabolism and lipid synthesis in liver. Biochimie. 87:81–86.
Publisher | Google Scholor - Gariani K,JornayvazFR. Pathophysiology of NASH in endocrine diseases. Endocrin Connect. 10: R52–R65.
Publisher | Google Scholor - Ritter MJ, Amano I, Hollenberg AN. (2020). Thyroid Hormone Signaling and the Liver. Hepatology. 72: 742–752.
Publisher | Google Scholor - Li Y, Wang L, Zhou L, Song Y, Ma S, et al. (2017). Thyroid stimulating hormone increases hepaticgluconeogenesis via CRTC2. Mol Cell Endocrinol, 446, 70–80.
Publisher | Google Scholor - Pihlajamaki, Boes T, Kim EY, Dearie F, Kim BW, Schroeder J, et al. (2009). Thyroidhormone-related regulation of gene expression in human fatty liver. J Clin Endocrinol Metab. 94: 3521–3529.
Publisher | Google Scholor - Araki O, Ying H, Furuya F, Zhu X, Cheng SY. (2005). Thyroid hormone receptor beta mutants: Dominant negative regulators ofperoxisome proliferator-activated receptor gamma action. Proc NatlAcad Sci USA 102: 16251–16256.
Publisher | Google Scholor - Rautou PE, Mansouri A, Lebrec D, Durand F, Valla D, et al. (2010). Autophagy in liver diseases. J Hepatol. 53:1123–1134.
Publisher | Google Scholor - Messarah M, Boumendjel A, Chouabia A, Klibet F, et al. (2010). Influence of thyroiddysfunction on liver lipid peroxidation and antioxidant status in experimental rats. Exp Toxicol Pathol 62:301–310.
Publisher | Google Scholor - Loria P, Carulli L, Bertolotti M, Lonardo A. (2009). Endocrine and liver interaction: The role of endocrine pathways in NASH. Nat Rev Gastroenterol Hepatol. 6: 236–247.
Publisher | Google Scholor - Parthasarathy G, Revelo X, Malhi H. (2020). Pathogenesis of Nonalcoholic Steatohepatitis: An Overview. Hepatol Commun. 4: 478–492.
Publisher | Google Scholor - Buzzetti E, Pinzani M ,Tsochatzis EA. (2016). The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 6:1038–1048.
Publisher | Google Scholor - Mansouri A, Gattolliat CH, Asselah T. (2018). Mitochondrial Dysfunction and Signaling in Chronic Liver Diseases. Gastroenterology. 155: 629–647.
Publisher | Google Scholor - Guilherme A, Virbasius JV, Puri V, Czech MP. (2008). Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 9: 367–377.
Publisher | Google Scholor - Asrih M, Altirriba J, Rohner-Jeanrenaud F, Jornayvaz F.R. (2015). Ketogenic Diet Impairs FGF21 Signaling and Promotes Differential Inflammatory Responses in the Liver and White Adipose Tissue. PLoS ONE 10, e0126364.
Publisher | Google Scholor - Montandon SA, Somm E, Loizides-Mangold U, de Vito C, Dibner C, Jornayvaz FR. (2019). Multi-technique comparison ofatherogenic and MCD NASH models highlights changes in sphingolipid metabolism. Sci Rep 9:16810.
Publisher | Google Scholor - Somm E, Jornayvaz FR. (2018). Fibroblast Growth Factor 15/19: From Basic Functions to Therapeutic Perspectives. Endocrin Rev. 39: 960–989.
Publisher | Google Scholor - Somm E, Henry H,Bruce SJ, Aeby S, Rosikiewicz M, Sykiotis GP, et al. (2017). beta-Klotho deficiency protects against obesity through a crosstalk between liver, microbiota, and brown adipose tissue. JCI Insight. 2, e91809.
Publisher | Google Scholor - Somm E, Montandon SA, Loizides-Mangold U, Loizides-Mangold U, Gaia N, Lazarevic V, et al. (2021). The GLP-1R agonist liraglutide limits hepatic lipotoxicity and inflammatory response in mice fed a methionine-choline deficient diet. Transl Res. 227: 75–88.
Publisher | Google Scholor - Bugianes E, Moscatiello S, Ciaravella MF, Marchesini G. (2010). Insulin resistance in nonalcoholic fatty liver disease. Curr Pharm Des.16:1941–1951.
Publisher | Google Scholor - Jornayva FR, Samuel VT, Shulman GI. (2010). The role of muscle insulin resistance in the pathogenesis of atherogenic dyslipidemia and nonalcoholic fatty liver disease associated with the metabolic syndrome. Annu Rev Nutr. 30 :273–290.
Publisher | Google Scholor - Krauss RM, Siri PW. (2004). Metabolic abnormalities: Triglyceride and low-density lipoprotein. Endocrinol Metab Clin N Am. 33: 405–415.
Publisher | Google Scholor - Ginsberg HN. (2000). Insulin resistance and cardiovascular disease. J Clin Investig. 106: 453–458.
Publisher | Google Scholor