Case Report
Stiff Person Syndrome Leading to Apnea: A Case Report
- Chiemi Kobayashi *
Japan Physical Therapy Association Home-based membership 286-0007・2-24-44 Hananokidai Narita Chiba, Japan.
*Corresponding Author: Chiemi Kobayashi, Japan Physical Therapy Association Home-based membership 286-0007・2-24-44 Hananokidai Narita Chiba, Japan.
Citation: Kobayashi C. (2024). Stiff Person Syndrome Leading to Apnea: A Case Report. Journal of BioMed Research and Reports, BioRes Scientia Publishers. 5(3):1-9. DOI: 10.59657/2837-4681.brs.24.109
Copyright: © 2024 Chiemi Kobayashi, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: August 14, 2024 | Accepted: August 30, 2024 | Published: September 09, 2024
Abstract
This report aimed to evaluate the causes of apnea during the treatment of stiff person syndrome (SPS), which can cause fatal dyspnea, and the possibility of respiratory physical therapy. I investigated a woman in her late 50s with classical SPS. Hypoxia and hypercapnia were observed upon the onset of apnea. The lung insufflation capacity (LIC) trainer effectively treated restrictive ventilatory dysfunction associated with trunk muscle stiffness; however, apnea persisted, and central alveolar hypoventilation was diagnosed. The patient presented with respiratory symptoms with spasms in SPS spectrum disorders. Recognizing that SPS can progress to fatal apnea is important, along with working with a pulmonologist to assess and manage changes in respiratory function over the long term, as in this case. The continued use of LIC trainers for respiratory physiotherapy should also be considered. This is one of the few reports detailing a long-term course.
Keywords: apnea; chest radiograph dynamic evaluation; diaphragmatic echo; lung insufflation capacity trainer; stiff person syndrome; transcutaneous gas analysis
Introduction
Stiff person syndrome (SPS) is a progressive autoimmune neurological disease with the main symptoms of muscle stiffness and painful muscle spasms during voluntary movements due to synaptic inhibition caused by GABAergic antibodies at the cerebral, medullary, the brain, brain stem, and spinal cord levels. Sound (noise), light, voluntary movements, cold stimuli, and emotional changes induce muscle spasms and stiffness. Furthermore, in Japan, respiratory dysfunction due to SPS has not been recognized beyond its characteristic symptoms. When muscle stiffness extends to the entire rib cage, dyspnea is present [1]. According to Daniela et al. [2], respiratory symptoms with spasms (RSwS) in SPS spectrum disorders can lead to serious morbidity and mortality if left untreated. This highlights the importance of patient complaints and interviews.
Respiratory dysfunction in severe neurologically intractable diseases can be broadly categorized into voluntary respiratory control disorder (diaphragm nerve/muscle disorder) and autonomic (central) respiratory control [3]. Furthermore, SPS involves stiffness of the chest, abdomen, and lumbar dorsal muscles. Restricted ventilation dysfunction due to muscle stiffness has been added to the category [1]. Furthermore, the high-dose long-term steroid treatment increased the possibility of a diaphragm neuromuscular disorder [4,5]. Dalakas et al. [6] also reported shortness of breath in 50% of the 20 patients with SPS. A previous study (n=18) [7] yielded similar results, with 50% of patients experiencing dyspnea. Sexauer et al. [1] reported that 88% of patients (n=17) had dyspnea. In the 2018 SPS hospitalization survey conducted across the United States by Crispo et al. [8], respiratory failure and cardiac arrest were observed in 1.7% of patients.
Respiratory dysfunction can have fatal outcomes. In a case report by Fujiya et al. [9], intravenously injecting diazepam to eliminate muscle stiffness showed an increase in vital capacity (VC) and maximum ventilation for restrictive ventilatory dysfunction thought to be caused by respiratory muscle stiffness. The ventilation reaction test revealed a normal hypoxic ventilation reaction. However, the ventilatory response to hypercapnia was abnormal. Furthermore, after 5 days of increasing diazepam to 60 mg/day, the patient stopped breathing and was resuscitated with a respirator, but died 9 days later.
When using large doses such as diazepam, it is necessary to pay attention to respiratory depression. Specifically, these patients were more likely to experience CO2 narcosis. Diaphragmatic dysfunction in SPS remains unknown [1]. Acute respiratory failure, which manifests as apneic episodes, is a life-threatening and unpredictable SPS complication [10]. However, its pathophysiology is not fully understood. The two suggested mechanisms are as follows: (1) apnea due to muscle rigidity and paroxysmal muscle spasms; and (2) paroxysmal autonomic hyperactivity. Patients with SPS have been reported to experience sudden and unexpected deaths, and all described cases have been associated with apnea. The onset of apnea during SPS should be considered a sign of possible progression towards acute respiratory failure and sudden death, and early immunotherapy should be initiated in such situations, including intravenous immunoglobulins as first-line treatment [10].
This report aimed to present the possibility of dyspnea progressing to fatal apnea syndrome, evaluate the causes of apnea from various perspectives based on evaluations conducted during treatment, and present the possibility of respiratory physical therapy.
Case and Course
The patient was a woman in her late 50s with classic SPS. Her medical history included asthma in middle school and from her 30s to the onset of the disease, for which she was receiving ongoing treatment with anti-allergy medications and inhaled steroids. Regarding her family history, her mother had IgA nephropathy and is on dialysis.
In May 2012, in her late 40s, she had myoclonus of the tongue and muscle cramps in her left upper leg. In May 2012, after receiving an orthopedic examination at T Hospital, she was referred to the Department of Neurology, N Hospital. Her muscle spasms and stiffness gradually progressed to both lower limbs and the right upper limb, and she was forced to use a cane outdoors. Initially, symptomatic treatment (sodium valproate tablets 1,200 mg/day, clonazepam tablets 1 mg/day, gabalon 15 mg/day, and valproic acid tablets 600 mg/day) was administered. Her dyspnea Numerical Rating Scale score was 10 during her exertion, and she had difficulty going up and down a flight of stairs. In 2016, she had an attack of stiffness all over her body while walking in a crowd and was taken to the hospital by ambulance, with difficulty breathing and severe pain (pseudoagoraphobia). Three courses of steroid pulse therapy were administered, leading to rapid improvement such that she could run quickly. Subsequently, due to the steroid-centered treatment, such as dexamethasone pulse, she had cataracts and steroid-induced diabetes mellitus; therefore, it was necessary to reduce the dose of steroids. Oral administration included valproic acid tablets 1,200 mg/day, clonazepam tablets 1 mg/day, baclofen 30 mg/day, diazepam tablets 2 mg/day, and prednisolone tablets, which were gradually reduced. In addition, following high-dose steroid administration, her weight gradually increased to a maximum of 69 kg, with a body mass index (BMI) of 30 kg/m2. Later, because of an increase in glycated hemoglobin to 8.0%, insulin therapy by a diabetologist was introduced in 2017. In 2018, bilateral femoral head necrosis occurred, which led to an attempt to wean herself off steroids. She was given immunoglobulin (IVIG) in the hospital; however, as her condition stabilized, IVIG was administered on an outpatient basis for 2 days in 5 weeks. In February 2019, she experienced acute respiratory failure (shadow in the lower left lung field) owing to cold-like symptoms. The patient was hospitalized for approximately 1 month. There were also asthma-like findings, and it was difficult to inhale bronchodilators, which disturbed sleep, as hypoxia affected night breathing. With the aim of reducing the amount of breathing work, oxygen administration was added at 2 L during the day (3 L during exercise) using a nasal cannula, non-invasive positive pressure ventilation (NPPV, Philips Society) was performed at night, and 2 L of oxygen were added using pressure ventilation. To maintain >92% oxygen saturation (SpO2), 15 backups were performed in S/T mode, and the patient was managed overnight with 4 hPa positive expiratory pressure (EPAP) and 10 hPa positive inspiratory airway pressure (IPAP).
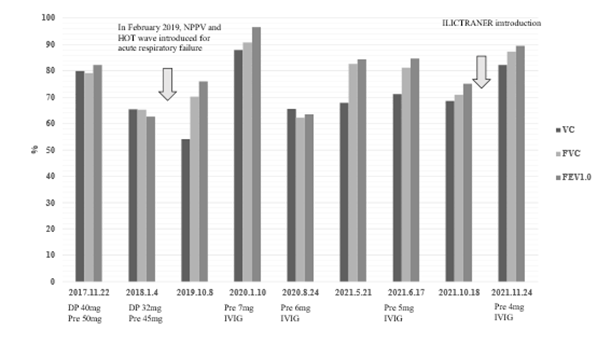
In September 2019, she was hospitalized for approximately 7 days with pneumonia. In October 2019, spirometry showed a decrease in %VC to 54.1%, %FVC to 70.2%, and FEV1.0 to 76.1 after pneumonia (Figure 1). On the same day, the BGA (inguinal artery) was 2 L of oxygen using a nasal cannula, with partial pressure of oxygen (PO2) of 68.2 mmHg and partial pressure of carbon dioxide (PCO2) of 45.6 mmHg. At this point, the internal impairment of respiratory function was judged to be grade 1, with both lower limbs rated at grade 2 and the upper limb at grade 6, resulting in combined into a physical disability certificate grade 1 (financial support and various social support can be received according to the severity of the illness of persons with disabilities in Japan). At the end of August 2020, she developed somnolence and was referred to a pulmonologist. At that time, her weight was 64.1 kg, with a BMI of 27.9 kg/m2, and according to the standards of the Japan Society of Obesity (JASSO), it was obesity level I [11].
Figure 1: Spirometry course (symptomatic treatment is conducted in a similar manner)
DP, decadron pulse; Pre, Prednisolone; IVIG, Immunoglobulin therapy; HOT, Home oxygen; NPPV, non-invasive positive pressure ventilation; LIC, lung insufflation capacity trainer.
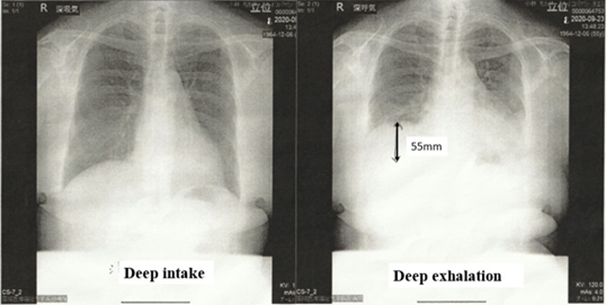
Initially, sleep apnea syndrome was tested on an outpatient basis, and the average SpO2 value was 97%, the minimum SpO2 was 88%, the maximum SpO2 was 100%, the minimum oxygen saturation level was 93%, and the American Academy of Sleep Medicine guideline reference value was pAHI=3.6. No hypoxemia was observed at this point. She was admitted to the pulmonology department for 1 week in September 2020 to be tested for arousal disorders. Neuromuscular was assessed using the time-resolved quantitative assessment of diaphragmatic movement in the dynamic evaluation of standing chest radiographs, as described by Hida et al. [12]. In healthy individuals, the diaphragmatic range of motion during deep breathing is 49.1 mm ± 17.0 mm and 52.1 ± 15.9 mm in the right and left diaphragm, respectively. If it falls below the lower limit of this value, it will be judged to be abnormal [12]. The patient’s right diaphragm had a range of motion of 55 mm (Figure 2), indicating that she had no problems with spontaneous respiratory function. However, images of pneumonia remained, and the boundaries of the left diaphragm were blurred; thus, it could not be measured. Continuous transcutaneous blood gas monitoring is a non-invasive, continuous method for estimating the partial pressure of arterial oxygen and partial pressure of arterial carbon dioxide and can be utilized in determining the suitability of NPPV and adjusting equipment, enabling central and receptor determination among patients with neuromuscular diseases [13].
Figure 2: Evaluation of diaphragm function via standing chest radiograph
On the left side, the diaphragm was unclear because of pneumonia effects; hence, the distance traveled to deep exhalation was measured using deep inspiration as a baseline for the right diaphragm, and it was 55 mm.
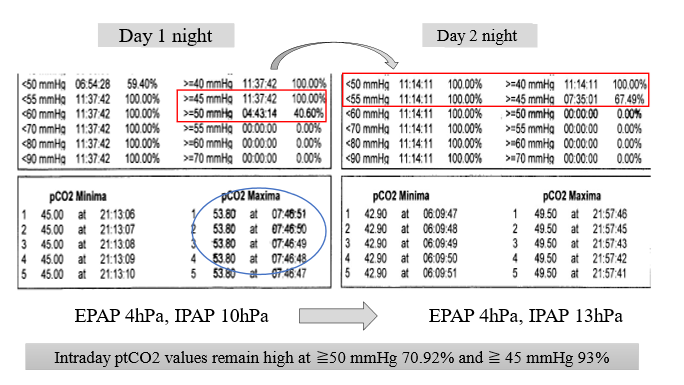
NPPV was loaded with 2 L of oxygen during the day and night, and the nighttime transcutaneous partial pressure of carbon dioxide (tcpCO2) before adjusting the intake pressure was 40.6% when it was ≥50 mmHg (red circle) and 100% when it was ≥45 mmHg; the highest tcpCO2 was in the morning (blue circle), which may explain why it was difficult for her to wake up. After maintaining EPAP at 4 hPa and adjusting IPAP from 10 hPa to 13 hPa, the tcpCO2 partial pressure decreased to ≥50mmHg and ≥45mmHg at 0% and 67.49%, respectively. However, daytime values remained high at 70.92% and 93.27% for tcpCO2 ≥50 mmHg and ≥45 mmHg, respectively (Figure 3).
Figure 3: Transdermal gas monitor progress EPAP, expiratory positive airway pressure; IPAP, inspiratory positive airway pressure
For 1 month from October 2021, the Pulmonary Ventilation Capacity Trainer (LICTRAINER) developed by Yorimoto [14] was used, which has been reported to be effective for amyotrophic lateral sclerosis (ALS). Spirometry showed that %VC 71.3% (VC; 1,820 mL) improved from restrictive dysfunction to the normal range of %VC 82.3% (VC; 2,180 mL) (Figure 1). The white area between the back-valve masks refers to the lung insufflation capacity trainer (LIC), and the upper pressure limit was set to 10 mmHg. SPS causes simultaneous contraction of motor and antagonist muscles. Hence, a slow expansion of a few seconds is required. Therefore, the patient sat comfortably on a sofa with a cushion placed on her knees to reduce the burden on her upper limbs while gradually increasing the number of pressurizations and lengthening inhalation time. She closed the left exhalation release valve with her left thumb and pressurized the inhalation with her right hand, repeating the process from a few times to gradually increasing the number of times, and finally, up to 10 inspiratory compressions (Video 1).
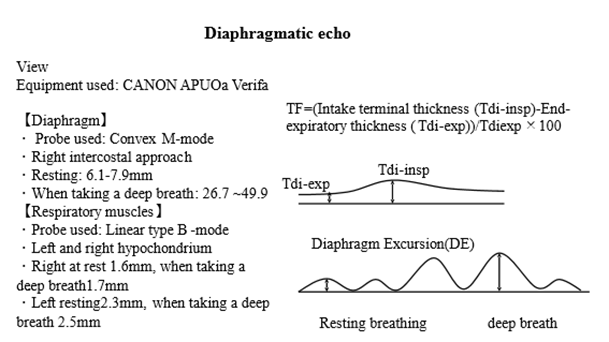
In May 2021, diaphragm muscle thickness and activity were measured using echocardiography (APLIO Verifia, Canon) (Figure 4). In patients with diaphragmatic dysfunction, the diaphragm becomes chronically atrophied and does not thicken during inspiration [15].
Figure 4: Diaphragm excursion and thickening fraction by diaphragmatic echo.
Modified Figure 4 of the diaphragmatic echo in the supine position of Laghi et al. [20].
TF: thickening fraction; DE: diaphragm excursion.
Evaluating the mobility of the posterior wall of the diaphragm is also helpful in measuring diaphragm muscle movement (distance traveled by the diaphragm) [15,16]. Diaphragmatic thickness was measured using a convex B-mode probe via the right intercostal approach. The diaphragmatic movement distance was measured using a linear B-mode probe in the left and right hypochondriacs [15].
Diaphragm thickness = (end inspiratory thickness (Tdi-insp) - end expiratory thickness (Tdi-exp)) × 100 = thickening fraction (TF): normal value ≥20% [15], the mean ± SD for normal diaphragm excursions (DE) for women was 15.1±3.7 mm at rest and 55.4±13.3 mm at deep breathing [16].
In her case, M-mode resting breathing, deep breathing, and TF were 6.1–7.9 mm, 26.7–49.9 mm, and 23%, respectively, which were within the normal range. In the B-mode hypochondrium, DE was 1.6 mm when the right was resting, 1.7 mm during deep breaths, 2.3 mm when the left was resting, and 2.5 mm during deep breaths, and the DE decreased on both sides (Figure 4) [15,16].
From mid-January 2022, nighttime SpO2 (less than 88%) began to drop, and an apnea alert was issued at the end of January. In early February, the alarm for apnea and minimum minute ventilation (1 L) began to sound 20 times, which disturbed sleep. In mid-February, the apnea alarm and the minute ventilation alarm began to sound up to 29 times. Furthermore, the patient had trouble waking up during the day and continued to experience abnormal conditions; she could not remain alert throughout the day.
Subsequently, she was admitted again for respiratory control. During that time, an unconscious decrease was observed in SpO2, with its levels dropping to 80% immediately after waking up; bradycardia (<50>180 beats/min) associated with tactile stimulation were also observed. Without NPPV pressure adjustment, she could change 2 L of oxygen to 4 L at night and maintain alertness, oxygenation, low tcpCO2, and tcpO2 at 92% using NPPV and 2 L of oxygen during the day. Therefore, the hypoxic ventilation reaction was also abnormal at this time. It was different from the hypercapnic ventilation response anomaly reported by Fujiya et al. [9].
In 2023, the patient was hospitalized owing to continuous seizures throughout her body. BGA upon admission was PO2 and PCO2 of 40.7 mmHg and 46.5 mmHg, respectively. Convulsive attacks of muscle spasm (a phenomenon where muscle stiffness of the whole body occurs persistently) were accompanied by hypoxia and hypercapnia, the respiratory rate was >44 times/min, and ventilation was almost impossible (Video 2).
When her activity level increased slightly, she experienced seizures and required intravenous diazepam. Spontaneous breathing was almost zero at night and frequently zero during the day, regardless of her level of alertness, resulting in a respiratory state dependent on NPPV. Steroid pulses were administered; however, there was no improvement. IVIG dramatically improved seizures, leading to an improvement in voluntary movements, which was subsequently addressed by shortening the interval between IVIGs. She could remain awake during the day; however, her apnea did not improve, and she was in a breathing state that relied on NPPV backup at night.
Discussion
According to the results of the dynamic assessment of the standing chest radiography and the thickness of the diaphragm, in her case, the large dose of steroids did not affect the neuromuscular activity of the diaphragm. Diaphragmatic echoes performed in the standing position correlated with BMI regarding the reduction in bilateral DE [17]. The weight during measurement was 61.5 kg, the BMI was 26.4 kg/m2, and JASSO obesity level I. Boussuges et al. [18] stated that it is essential to evaluate patients with high BMI or respiratory dysfunction in a sitting position. The decrease in DE was thought to be due to impaired respiratory function and mild obesity to which NPPV was administered. In this case, the spontaneous respiratory control of the diaphragmatic function was effective as long as the patient was not fully awake and in the supine position; however, central problems such as central receptors and chemoreceptors were considered to be the main factors. If a patient has respiratory dysfunction similar to hers and has mild obesity, a diaphragm echogram should be performed in a sitting position and compared with spirometry.
Abnormal wakefulness during the day with transcutaneous gas monitoring revealed CO2 narcotics associated with apnea. However, not all SPS leads to respiratory failure. Nevertheless, it should be noted that classic SPS is a disease type that is more likely to cause respiratory problems.
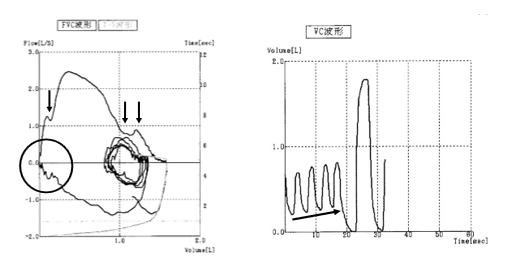
In rehabilitation, physiotherapy for respiratory muscle strengthening is not recommended because spirometry frequently shows contractions occurring simultaneously during breathing in patients with SPS. In the FVC waveform, the area surrounded by arrows and circles indicates inspiratory and exhalatory disorders due to the simultaneous contraction of the motor and antagonist muscles. The diagonal arrows of the VC waveform do not change the tidal volume; however, the residual air volume gradually increased
(Figure 5). The LICTRAINER for thoracic expansion and diaphragm mobility may be effective in improving restrictive ventilatory dysfunction in these patients. Compared with the report on patients with ALS by Yorimoto et al. [18], this method may be effective because it shows that the group that can measure VC but cannot measure the maximum air flow capacity (MIC) has improved to the group that can also measure MIC. Continuing with LICTRAINER may allow individuals to maintain lung capacity against the pressure of NPPV.
In her case, immunotherapy, including IVIG, was already in place, and I consider it essential to adjust the duration of IVIG implementation to RSwS because NPPV is essential for controlling central respiratory function associated with apnea.
Figure 5: Characteristics of spirometry
VC, vital capacity; FVC, Forced Vital Capacity
A reversal was observed for VC (65.5 %) and FVC (62.2 %).
Conclusion
According to previous studies [1], dyspnea is found in half of patients with SPS, with a high possibility of progression to respiratory dysfunction. I have to be concerned that an unusual wakefulness disorder, such as in this case, may reflect serious respiratory problems. Lung capacity measurements are not sensitive in detecting early respiratory neuromuscular lesions [18]; hence, if spirometry detects a decline, the implementation of respiratory medicine intervention and respiratory physiotherapy (LICTRAINER) [19] should be considered. In addition, in this case, the need to take preventive measures to avoid severe seizures is emphasized since the response to hypoxemia and hypercapnia is poor. Because of its ease of use and low burden on the patient, it is believed that diaphragmatic echo graphic evaluation is particularly effective in differentiating central alveolar hypoventilation. I hope that this report will significantly contribute to the evaluation and treatment of patients with SPS. In this case, RSwS showed a dramatic improvement in IVIG; therefore, continued IVIG is essential.
Declarations
Acknowledgments
I would like to thank Editage (www.editage.jp) for English language editing. I collected the data and prepared figures. I would also like to thank Dr. Yuriko Yoshikawa, Department of Neurology, Narita Red Cross Hospital, for providing the clinical findings and the hospital’s director, Nobuyuki Aotuka, for kindly providing the materials. Clinical examinations were conducted by a chest radiograph technician and a clinical laboratory technician under the direction of the physician. The transcutaneous gas analysis was conducted by a clinical engineer.
Competing interest declaration
There are no conflicts of interest to disclose in this research.
Ethical consideration
Since this was a retrospective case report, I was exempt from the ethical review of the International University of Health and Welfare, to which I belonged at the time. Treatment evaluation is a category of medical care, and the physical and mental burden on the patient is minimal. Consent to submit the manuscript has been obtained.
Funding
The author reports no funding.
References
- Sexauer, W., Woodford, M., Pack, K., Allen, A., Crawford, A., & Rakocevic, G. (2019). Dyspnea in patients with stiff-person syndrome. American Journal of Medical Sciences, 358(4):268-272.
Publisher | Google Scholor - Pimentel Maldonado, D. A., Balshi, A., Hu, C., Fitzgerald, K. C., Koshorek, J., Reyes-Mantilla, M. I., Obando, D., Wang, Y., & Newsome, S. D. (2023). Respiratory symptoms are common in stiff person syndrome spectrum disorders and are associated with the number of body regions involved. European Journal of Neurology, 30(8):2498-2505.
Publisher | Google Scholor - Racca, F., Vianello, A., Mongini, T., Ruggeri, P., Versaci, A., Vita, G. L., & Vita, G. (2020). Practical approach to respiratory emergencies in neurological diseases. Neurological Sciences, 41(3):497-508.
Publisher | Google Scholor - Dekhuijzen, P. N. R., & Decramer, M. (1992). Steroid-induced myopathy and its significance to respiratory disease: A known disease rediscovered. European Respiratory Journal, 5(8):997-1003.
Publisher | Google Scholor - Ikutomo, M., Tamaki, T., Tannba, M., Misina, T., Shimo, S., & Muramatu, K. (2016). The effect of hyperglycemia on phrenic motoneurons: A preliminary study focusing on motoneuron number. Movement Disorders, 26:49-52.
Publisher | Google Scholor - Dalakas, M. C., Fjii, M., Li, M., & McElroy, B. (2000). The clinical spectrum of anti-GAD antibody-positive patients with stiff-person syndrome. Neurology, 55:1531-1535.
Publisher | Google Scholor - Chiemi, K., Yukiko, M., & Yusuke, N. (2023). Quality of life of patients with stiff person syndrome. Clinical Case Reports and Studies, 2:1-8.
Publisher | Google Scholor - Crispo, J. A. G., Thibault, D. P., Fortin, Y., & Willis, A. W. (2018). Inpatient care for stiff person syndrome in the United States: A nationwide readmission study. Journal of Clinical Movement Disorders, 5:1-8.
Publisher | Google Scholor - Fujiya, S., Minohara, O., Kawakam, Y., Shida, A., Asanuma, Y., Yoshikawa, T., Mikami, H., Osaki, S., & Murao, M. (1981). Characteristics of respiratory physiology in a case of stiff-man syndrome. Journal of the Japanese Society of Thoracic Diseases, 19:881-884.
Publisher | Google Scholor - Vincent, J., Laurent, L., Thierry, G., Henry, C., Daniel, D. S., & Etienne, dR. (2016). Acute respiratory failure in a patient with stiff-person syndrome. Neurocritical Care, 25(3):455-457.
Publisher | Google Scholor - Ogawa, W., & Miyazaki, S. (2015). Diagnosis criteria for obesity and obesity disease. HEP, 42:301-306.
Publisher | Google Scholor - Hida, T., Yamada, Y., Ueyama, M., Araki, T., Nishino, M., Kurosaki, A., Jinzaki, M., Honda, H., Hatabu, H., & Kudoh, S. (2019). Time-resolved quantitative evaluation of diaphragmatic motion during forced breathing in a health screening cohort in a standing position: Dynamic chest phrenicography. European Journal of Radiology, 113:59-65.
Publisher | Google Scholor - Kakuchi, T., & Takeuti, M. (2017). Respiratory management device: Transcutaneous gas monitor. Japanese Journal of Respiratory Care, 34:149-153.
Publisher | Google Scholor - Yorimoto, K. (2019). Current status of LICTRAINER. Incurable Diseases and Home Care, 25:31-33.
Publisher | Google Scholor - Andrea, J. B., Caittin, J. B. A., Leili, S. G., Jeffrey, A, S., James, C. W., Eric, J. S. (2012). Two-dimensional ultrasound imaging of the diaphragm: Quantitative values in normal subjects. Muscle & Nerve, 47(6): 884-889.
Publisher | Google Scholor - Santana, P. V., Cardenas, L. Z., Albuquerque, A. L. P., Carvalho, C. R. R., & Caruso, P. (2020). Diaphragmatic ultrasound: A review of its methodological aspects and clinical uses. Journal of the Brazilian Society of Pneumology, 46(6):1-17.
Publisher | Google Scholor - Boussuges, A., Finance, J., Chaumet, G., & Bregeon, F. (2021). Diaphragmatic motion recorded by M-mode ultrasonography: Limits of normality. ERJ Open Research, 7(1):1-8.
Publisher | Google Scholor - Walter, V., Murray, G. E., & Manue, L. G. C. (1987). Determinants of respiratory muscle weakness in stable chronic neuromuscular disorders. The American Journal of Medicine, 82(1):53-58.
Publisher | Google Scholor - Keisuke, Y., Ariake, Y., Saotome, T., Mori-Yoshimura, M., Tadashi, T., Yuji, T., & Yoko, K. (2020). Lung insufflation capacity with a new device in amyotrophic lateral sclerosis: Measurement of the lung volume recruitment in respiratory therapy. Progress in Rehabilitation Medicine, 5:1-8.
Publisher | Google Scholor - Laghi, F. A. Jr., Saad, M., & Shaikh, H. (2021). Ultrasound and non-ultrasound imaging techniques in the assessment of diaphragmatic dysfunction. BMC Pulmonary Medicine, 21(1):85.
Publisher | Google Scholor