Voltammetry, NIRS Lab, Medicine Center, Verona, It
SSRI and COX-2 Inhibitor Combination: In Vivo Studies of Mechanism of Action
- Francesco Crespi *
Voltammetry, NIRS Lab, Medicine Center, Verona, Italy.
*Corresponding Author: Francesco Crespi, Voltammetry, NIRS Lab, Medicine Center, Verona, Italy.
Citation: Crespi F. (2024). SSRI and COX-2 Inhibitor Combination: In Vivo Studies of Mechanism of Action, Journal of Neuroscience and Neurological Research, BioRes Scientia Publishers. 3(1):1-12. DOI: 10.59657/2837-4843.brs.24.014
Copyright: © 2024 Francesco Crespi, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: January 30, 2024 | Accepted: March 25, 2024 | Published: April 09, 2024
Abstract
Recent preclinical studies have suggested that inhibitors of COX-2 such as lumiraCOXib or nimesulide have antidepressant effects in animal models. The role of serotonin (5-HT) in major depressive disorder (MDD) has been studied since long ago and subsequent development of selective serotonin reuptake inhibitors (SSRIs) has introduced these compounds as first choice treatment for depressed patients. The combination of SSRI/COX-2 inhibitor is a recent dual pharmacology approach to treating major depression and it is postulated that COX-2 inhibition may result in altered neuronal firing and consequent neurotransmitter release in associated terminal field regions. Here, in order to examine this assumption, in vivo voltammetry associated with in vivo electrophysiology is applied at micro-biosensors stereotaxically implanted in the relevant brain areas where neurotransmitter release and neuronal firing can be measured simultaneously in real time before and after acute treatment in anaesthetized rodents.
Keywords: SSRI; COX-2 inhibition; in vivo voltammetry; in vivo electrophysiology; serotonin
Introduction
The role of serotonin (5-HT) in major depressive disorder (MDD) was postulated in the sixties [1] and inhibition of serotonin reuptake to enhance stimulation of postsynaptic serotonin receptors was among the proposed healing approaches. This opened the way to the development of selective serotonin reuptake inhibitors (SSRIs). SSRIs are characterised by broad-spectrum effectiveness and high tolerance therefore used as first choice treatment for depressed patients, however a delayed onset of efficacy of 2–3 weeks in length is frequently observed [2]. More recently, it has been observed that anti-inflammatory drugs can generate antidepressant effects in clinical patients and positive effect in patients with psychiatric disorders; i.e., clinical trials on the cyclooxygenase (COX)-2 inhibitor celeCOXib revealed therapeutic effects in major depression [3] and that not only in single studies but also in meta-analyses. [4 for a review see ref 4]. Besides, preclinical studies have suggested that inhibitors of COX-2 such as lumiraCOXib or nimesulide have antidepressant effects in animal models. [5, 6]. The combination of SSRI/COX-2 inhibitor is a recent dual pharmacology approach to treating major depression, and published data on depressed patients suggest that the combination of cyclooxygenase-2 (COX-2) inhibition would improve the clinical outcome of the specific serotonin inhibitor (SSRI) paroxetine by alleviating depression symptoms of patients resistant to classical treatment [7, 8].
This has supportive pre-clinical data derived from a chronic escape deficit model of depression [9] and showing significant acceleration of the onset of action of fluoxetine via association with acetylsalicylic acid; a mixed COX inhibitor [10]. COX-2 enzymes are involved in the metabolism of arachidonic acid to produce prostaglandins [11]; these autacoid lipids prolong homeostatic functions and mediate pathogenic mechanisms, such as the inflammatory response. [12]. It is known that prostaglandin receptors (EP3) are co-localised in the dorsal raphe nucleus (RDN) in serotonergic cell bodies and in the locus coerulus in noradrenergic cell bodies. [13]. These EP3 receptors regulate neuronal firing by causing membrane depolarisation after agonist stimulation, thereby affecting neurotransmitter release [14, 15]. It is postulated that COX-2 inhibition will therefore triggers less prostaglandin induced activation of EP3 receptors to cause disinhibition of neuronal firing and increase neurotransmitter release in associated terminal field regions. [16, 17]. Considering that SSRIs also act at the 5-HT transporter and indirectly affect neurotransmission [18, 19] it is hypothesized that the pre-clinical and clinical effects of SSRI/COX-2 inhibitor combination are mediated centrally via acting upon neurotransmitter release controlled by neuronal firing to give an enhanced response.
To examine this supposition in vivo voltammetry associated with in vivo electrophysiology is applied at micro-biosensors stereotaxically implanted in the relevant brain areas where neurotransmitter release and neuronal firing can be measured simultaneously in real time before and after acute treatment in anaesthesised rats [20-25]. In particular, measurement of the firing rate of neurons lying in the RDN concomitantly with the release of 5-HT in the RDN projection structure namely the medial prefrontal cortex (mPFC) in rats injected with the COX-2 inhibitor 641784 [26], the SSRI paroxetine or a combination of both have been performed. 641784 has been shown to pass the blood–brain barrier [26] and dosages of 641784 were selected accordingly to studies performed in clinical investigations [27] starting with 1mg which should be with low or no efficacy up to 10mg. Similar approach was achieved for paroxetine [28].
Methods
The methodology of associated voltammetry and electrophysiology in vivo has been described earlier [20-25]. Briefly, voltammetry associated with electrophysiology has been performed at micro-biosensors stereotaxically implanted in the relevant brain areas so that neurotransmitter release and neuronal firing can be measured simultaneously in vivo, in situ, in real time before and after acute treatment in anaesthetised rats. In particular, the present experiments have been achieved by means of with micro-biosensors implanted in raphe dorsalis nucleus (RDN) to monitor cell firing and in prefrontal cortex (pFCx) for concomitant amperometric measurement of serotonin (5-HT) release.
Animals
Experiments on animals have been done on Male Sprague-Dawley CD rats (250 g, Charles River, Italy). The animals were housed four per cage, fed with Purina Cow with water available ad libitum and kept in a temperature-controlled environment. The experimental procedures were in line with the NIH Guidelines for Small Animal Research and approved by local review committees.
Study Design
Each rat was anaesthetized using urethane (1.4 g/kg i.p.), placed on a stereotaxic apparatus (D. Kopf, USA). For following stereotaxic preparation, micro-sensors preparation and their insertion in relevant brain regions under stereomicroscopy see ref 20-25. Successively, concomitant amperometric and electrophysiology measurement (continuous real time analysis) were performed as described [20-25] and after 30min of control/control measurements, treatments are performed with i.e., vehicle (control animals) or relevant compound(s), n=4 each group. The number of animals has been decided based upon the 3Rs, i.e., Reduction in the number of animals required; Refinement of the methodologies of analysis; Respect of the animals i.e., reducing their suffering as described [29].
The effects of 641784 1mg/kg or 10mg/kg s.c alone on amperometric and electrophysiological signals have been investigated. Then those of 641784 1mg/kg or 10mg/kg s.c associated to paroxetine 5mg/kg s.c. were also analysed. In the subsequently experiments 641784 10 mg/kg s.c. and paroxetine 5 mg/kg s.c. were used.
Sample type
1) Amperometric current levels of extracellular 5-HT measured in nanoAmperes (nA).
2) Electrophysiological spikes/sec as indication of cell firing.
Measure: Real time concomitant detection of amperometric changes of basal levels of extracellular 5-HT at terminal levels Prefrontal cortex (pFCx) and electrophysiological cell firing at cell bodies level i.e.raphe dorsalis (RDN).
Structure: Prefrontal cortex (and raphe dorsalis), coordinates: AP3.2 (-7.8), ML0.5 (0), DV4.6 (6.4) [Paxinos 1986].
Results expression: Percentage (%) of control; Statistical analysis details: 2w ANOVA and Dunnett.
Results
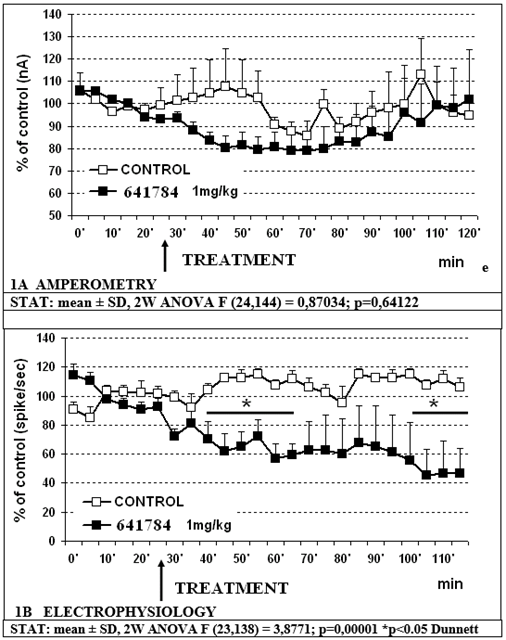
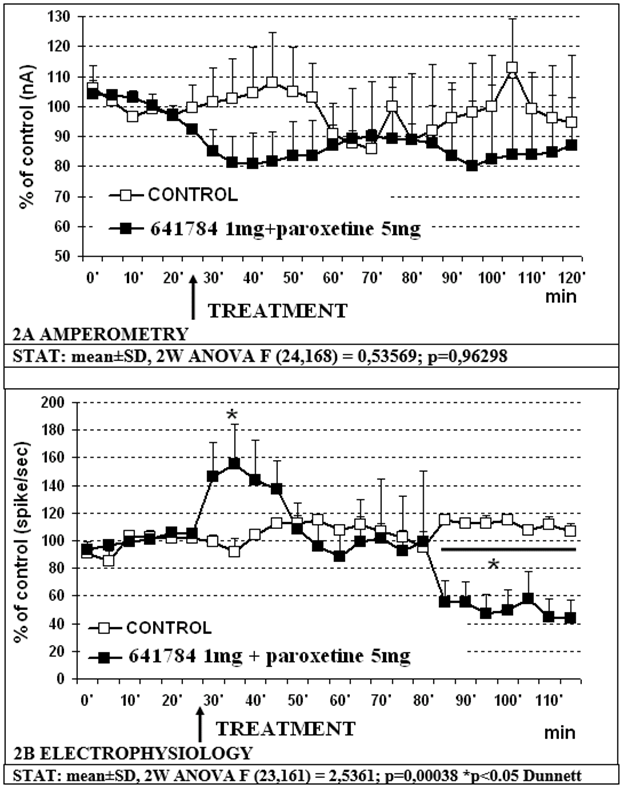
Data shown in figure 1A indicate that 641784 1mg/kg s.c. alone does not significantly affect the release of 5-HT in the mPFC with non-significant transient decrease to approx. 80% of controls. Concomitant cell firing in RDN shows a significant decrease (p less than 0.05) to approximately 60% within 30min and to approx. 44% of control values within 80min post treatment (figure 1B). Similarly, the treatment with the association 641784 1mg/kg paroxetine 5 mg/kg (s.c.) does not change significantly 5-HT release in prefrontal cortex with again a non-significant transient decrease to approx. 80% of control value (see figure 2A). Conversely, the electrophysiological analysis shows a biphasic modulation of cell firing in RDN with an initial increase up to a top of approx. 158% of controls within 10min, followed by a return to control values 20min later and a successive significant (p less than 0.05) decrease to approx. 50% of controls 55min later (figure 2B). In the successive experiments 641784 10 mg/kg s.c. and paroxetine at the dose of 5 mg/kg s.c. were used. In particular:
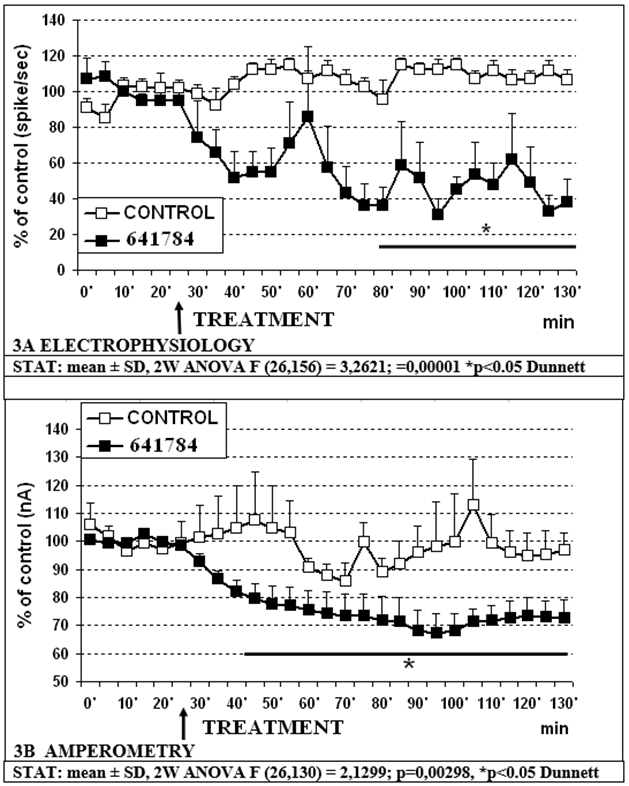
- -641784 10mg/kg s.c. causes a significant decrease in RDN firing to approximately 33% of controls within 70-90min and in 5-HT release in prefrontal cortex to approximately 68% of controls within 70min. (figure 3A and 3B, respectively).
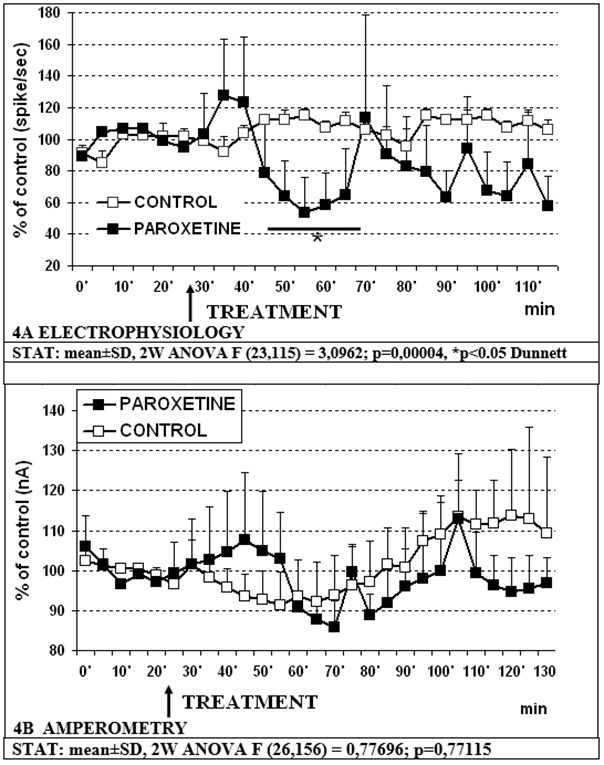
- -Paroxetine 5mg/kg s.c. causes a significant decrease in RDN cell firing to approximately 53% of controls within 25 min with a trend to an increase in 5-HT release in prefrontal cortex as previously observed [30, 31, 32] (figure 4A and 4B, respectively).
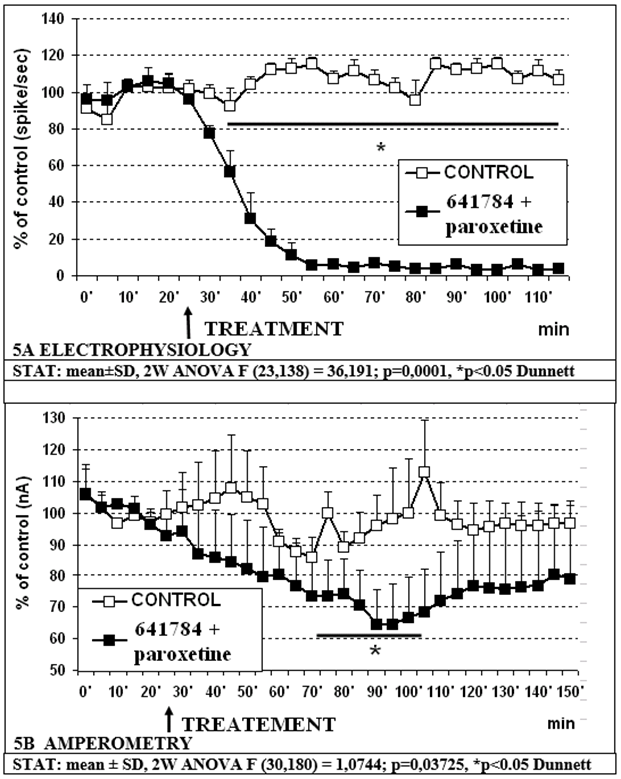
- -The combination of 641784 10mg/kg and Paroxetine 5mg/kg s.c. is resulting in a further decrease of cell firing in RDN which is reduced to approximately 3% of controls within 25-30min (figure 5A). In contrast, the effect of this association upon 5-HT release in pPFC does not change compared to the distinct effect of 641784 10 mg/kg alone (see figures 4A and 5B).
Figure 1: Effect of 641784 1mg/kg alone on in vivo amperometric (1A) and electrophysiological measurements (1B), respectively.
Figure 2: Effect of 641784 1mg/kg + paroxetrine 5mg/kg s.c. on amperometric (2A) and electrophysiological (2B) measurements, respectively.
Further Stats show no significant differences between:
- Treatment: 641784 1mg versus paroxetine 5mg/kg (F (24,144) =0,55832; p=0,95167);
- Treatment: 641784 1mg + paroxetine 5mg/kg versus 641784 1mg/kg (F (24,168) = 0,30384; p=0,99941);
- Treatment: 641784 1mg+paroxetine 5mg/kg versus paroxetine 5mg/kg (F (24,168) =0,61066; p=0,92210).
Figure 3: Effect of 641784 10mg/kg + paroxetine 5mg/kg s.c. on electrophysiological (3A) and amperometric (3B) measurements, respectively.
Figure 4: Effect of paroxetine 5mg/kg s.c. on electrophysiological (4A) and amperometric (4B) measurements, respectively.
Figure 5: Effect of 641784 10mg/kg + paroxetine 5mg/kg s.c. on electrophysiological (5A) and amperometric (5B) measurements, respectively.
Discussion
Recent works have proposed that the combination of cyclooxygenase-2 (COX-2) inhibition would improve the clinical outcome of the specific serotonin inhibitor (SSRI) paroxetine by alleviating depression symptoms of MDD patients resistant to classical treatment [33, 34]. However, findings from animal and human studies are contradictory. Some studies found adjunctive antidepressant effects for the selective COX-2 inhibitor celeCOXib [35,36,37,38,39]. In contrast, preclinical experiments indicate that the joint celecoxib treatment shows no-synergistic antidepressant effect to fluoxetine in a rat model of depression, while it could result in worsening the hypothalamic–pituitary–adrenal axis hyper activation [40]. Furthermore, while treatment whit SSRIs may rise synaptic serotonin, it is resulting in overall reduction of serotonin synthesis in the brain, in particular following long-lasting therapy with SSRIs. [41]. And this may explain the reduction in time of the related action upon depression. Again, the entire group of selective COX-2 inhibitors showed an increased mortality risk and no adjunctive antidepressant treatment effects [42].
Nevertheless, a recent meta-analysis from clinical trials on anti-inflammatory treatment for depressive symptoms supports a proof-of-concept concerning the use of anti-inflammatory treatment in depression [43].
In particular, it is postulated that COX-2 inhibition is influencing activation of EP3 receptors resulting in disinhibition of neuronal firing and increase neurotransmitter release in associated terminal field regions. [16, 17]. If one relates this with the fact that SSRIs also act at the 5-HT transporter and indirectly affect neurotransmission [18, 19] it can be hypothesized that the pre-clinical and clinical effects of SSRI/COX-2 inhibitor combination may be mediated centrally by influencing neurotransmitter release controlled by neuronal firing to give an enhanced response. To examine this supposition the present in vivo voltammetry associated with in vivo electrophysiology experiments indicates that:
- 641784 1mg/kg s.c. alone or associated to paroxetine 5mg/kg s.c. does not reduce significantly 5-HT release in prefrontal cortex accordingly to data presented earlier following neurochemical HPLC measurements [27] (see figures 1A, 2A).
- Concomitant electrophysiological measurements show that 641784 1mg/kg s.c. alone reduces significantly cell firing in RDN (figure 1B) and such an effect is obtained more rapidly with higher outcome when treating with 641784 10mg/kg s.c. alone (figure 3A). In addition, 641784 10mg/kg s.c. causes a significant decrease in 5-HT release in prefrontal cortex (figure 3B).
- Paroxetine alone 5mg/kg s.c. causes a significant decrease in RDN cell firing with a trend to an increase in 5-HT release in prefrontal cortex (figures 4A, 4B) as previously described [30 31, 32].
- Treatment with the association 641784 1mg/kg – paroxetine 5mg shows a biphasic modulation of cell firing in RDN when compared to treatment with 641784 1mg/kg s.c. alone that could at the end resume in no significant overall effect on such parameter (see figures 1B, 2B). A similar trend is possibly observable in figures 2B and 4A, that could indicate a contrasting effect of paroxetine upon 641784 1mg when monitoring RDN firing.
- However, combining 641784 10mg and paroxetine 5mg causes a significant, synergistic, prolonged decrease in DRN cell firing as compared to vehicle (control) and paroxetine alone (partial effect with respect to 641784 alone) (see figures 4A, 5A). Furthermore, this combination causes a significant reduction in 5-HT release compared to paroxetine alone (figures 4B, 5B). No difference is observed with respect to 641784 alone, although the variability is more pronounced within the combination (figures 3B, 5B).
Conclusion
In conclusion, these data indicate that 641784 reduces 5-HT release in prefrontal cortex, accordingly to data obtained with lower doses in neurochemical HPLC measurements [27]. Additionally, the combination 641784 + paroxetine shows a synergistic effect on RDN cell firing but not (yet) on 5-HT release in such acute conditions. This may anyway suggest that in chronic treatments the SSRI/COX-2 inhibitor(s) combination can give an enhanced response compared to SSRI alone via a specific action upon neuronal firing.In order to analyse this eventuality further work will be done in chronically treated conscious freely moving animals prepared for combined telemetric electrochemical-electrophysiological analysis as described [45, 46].
References
- Shaw D.M., Camps F.E., Eccleston E.G. (1967). 5-Hydroxytryptamine in the hindbrain of depressive suicides. Br. J. Psychiatry J. Ment. Sci. 113:1407-1411.
Publisher | Google Scholor - National Collaborating Centre for Mental Health. Depression: The Treatment and Management of Depression in Adults British Psychological Society; Leicester, UK: 2010.
Publisher | Google Scholor - Müller N., Schwarz M.J., Dehning S., Douhe A., Cerovecki A., Goldstein-Müller B., Spellmann I., Hetzel G., Maino K., Kleindienst N., et al. (2006). The cyclooxygenase-2 inhibitor celeCOXib has therapeutic effects in major depression: Results of a double blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol. Psychiatry. 11:680-684.
Publisher | Google Scholor - Müller, N. (2019). COX-2 inhibitors, aspirin, and other potential anti-inflammatory treatments for psychiatric disorders. Frontiers in Psychiatry, 10, 375.
Publisher | Google Scholor - Morgan A., Kondev V., Bedse G., Baldi R., Marcus D., Patel S. (2019). Cyclooxygenase-2 inhibition reduces anxiety-like behavior and normalizes enhanced amygdala glutamatergic transmission following chronic oral corticosterone treatment. Neurobiol. Stress. 11:100190.
Publisher | Google Scholor - Luo W., Luo Y., Yang J. (2020). Proteomics-based screening of the target proteins associated with antidepressant-like effect and mechanism of nimesulide. Sci. Rep. 10:11052.
Publisher | Google Scholor - Kopschina Feltes, P., Doorduin, J., Klein, H. C., Juárez-Orozco, L. E., Dierckx, R. A., Moriguchi-Jeckel, C. M., & de Vries, E. F. (2017). Anti-inflammatory treatment for major depressive disorder: implications for patients with an elevated immune profile and non-responders to standard antidepressant therapy. Journal of Psychopharmacology, 31(9), 1149-1165.
Publisher | Google Scholor - Roman, M., & Irwin, M. R. (2020). Novel neuroimmunologic therapeutics in depression: a clinical perspective on what we know so far. Brain, behavior, and immunity, 83, 7-21.
Publisher | Google Scholor - Guo, Q. H., Tong, Q. H., Lu, N., Cao, H., Yang, L., & Zhang, Y. Q. (2018). Proteomic analysis of the hippocampus in mouse models of trigeminal neuralgia and inescapable shock-induced depression. Neuroscience Bulletin, 34, 74-84.
Publisher | Google Scholor - Brunello, N., S. Alboni, G. Capone, C. Benatti, J. M. Blom, F. Tascedda, et al. (2006). Acetylsalicylic acid accelerates the antidepressant effect of fluoxetine in the chronic escape deficit model of depression. Int. Clin. Psychopharmacol. 21, 219-225.
Publisher | Google Scholor - Rajakariar, R., Yaqoob, M. M., & Gilroy, D. W. (2006). COX-2 in inflammation and resolution. Molecular interventions, 6(4), 199.
Publisher | Google Scholor - Ricciotti, E., & FitzGerald, G. A. (2011). Prostaglandins and inflammation. Arteriosclerosis, thrombosis, and vascular biology, 31(5), 986-1000.
Publisher | Google Scholor - Ek, M., Arias, C., Sawchenko, P., & Ericsson‐Dahlstrand, A. (2000). Distribution of the EP3 prostaglandin E2 receptor subtype in the rat brain: Relationship to sites of interleukin -1-induced cellular responsiveness. Journal of Comparative Neurology, 428(1), 5-20.
Publisher | Google Scholor - Bär, K. J., Natura, G., Telleria-Diaz, A., Teschner, P., Vogel, R., Vasquez, E. Ebersberger, A. (2004). Changes in the Effect of Spinal Prostaglandin E2 during Inflammation: Prostaglandin E (EP1–EP4) Receptors in Spinal Nociceptive Processing of Input from the Normal or Inflamed Knee Joint. The Journal of Neuroscience, 24(3), 642-651.
Publisher | Google Scholor - Jang, Y., Kim, M., & Hwang, S. W. (2020). Molecular mechanisms underlying the actions of arachidonic acid-derived prostaglandins on peripheral nociception. Journal of Neuroinflammation, 17, 1-27.
Publisher | Google Scholor - Liang, X., Wu, L., Wang, Q., Hand, T., Bilak, M., McCullough, L., & Andreasson, K. (2007). Function of COX-2 and prostaglandins in neurological disease. Journal of Molecular Neuroscience, 33, 94-99.
Publisher | Google Scholor - Kawahara, K., Hohjoh, H., Inazumi, T., Tsuchiya, S., & Sugimoto, Y. (2014). Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochimica et Biophysica Acta, 1851(4), 414-421.
Publisher | Google Scholor - Pehrson, A. L., & Sanchez, C. (2014). Serotonergic modulation of glutamate neurotransmission as a strategy for treating depression and cognitive dysfunction. CNS spectrums, 19(2), 121-133.
Publisher | Google Scholor - Dale, E., Pehrson, A. L., Jeyarajah, T., Li, Y., Leiser, S. C., Smagin, G., Sanchez, C. (2016). Effects of serotonin in the hippocampus: how SSRIs and multimodal antidepressants might regulate pyramidal cell function. CNS spectrums, 21(2), 143-161.
Publisher | Google Scholor - Crespi F. (2002). In vivo voltammetry and concomitant electrophysiology at a single biosensor to analyse ischaemia, depression and drug dependence. J Neurosci Methods, 119: 173-184.
Publisher | Google Scholor - Crespi F. (1996) Concomitant in vivo electrophysiological and voltammetric analysis indicate that ascorbic acid is a biochemical index of early ischaemia. Neuroscience Letters 215, 189-192.
Publisher | Google Scholor - Crespi F, Martin KF, Marsden CA. (1998). Measurement of extracellular basal levels of serotonin in vivo using nafion-coated carbon fibre electrodes combined with differential pulse voltammetry, Neuroscience, 27, 885-896.
Publisher | Google Scholor - Crespi F. (2010). SK channel blocker apamin attenuates the effect of SSRI fluoxetine upon cell firing in dorsal raphe nucleus: A concomitant electrophysiological and electrochemical in vivo study reveals implications for modulating extracellular 5-HT. Brain Research, 1334, 1-11.
Publisher | Google Scholor - Crespi F. (2018). Concomitant Behavioral, Electrochemical and Electrophysiological Study in Real Time on the Role of CRF and CRF Antagonist(s) in Anxiety and Depression: Possible Association CRF + 5-HT Receptor Antagonists? Int J Brain Disord Treat, 4:022.
Publisher | Google Scholor - Crespi F. (2019). Concomitant in Vivo Voltammetric and Electrophysiological Analysis Indicate that Nociceptin/Orphanin FQ Affects Dopamine and then Serotonin Activities in Brain Substancia Nigra. International Physiology Journal, 2(3), 1-9.
Publisher | Google Scholor - Gunn RN, Summerfield SG, Salinas CA, Read KD, Guo Q, Searle GE, Parker CA, Jeffrey P, Laruelle M. (2012). Combining PET biodistribution and equilibrium dialysis assays to assess the free brain concentration and BBB transport of CNS drugs. J Cereb Blood Flow Metab, 32(5):874-83.
Publisher | Google Scholor - Bloomer, J., Beaumont, C., Dear, G. J., North, S., & Young, G. (2016). The value of metabolite identification and quantification in clinical studies. Some case studies enabling early assessment of safety in humans: GlaxoSmithKline. Metabolite Safety in Drug Development, 275-291.
Publisher | Google Scholor - Bourin, M., Chue, P., & Guillon, Y. (2001). Paroxetine: a review. CNS drug reviews, 7(1), 25-47.
Publisher | Google Scholor - Crespi F, Congestri F, Formenti F. (2019). Near infrared spectroscopy (NIRS) to attain reduction-refinement–respect, the three Rs towards ANIMAL WELFARE in preclinical research. Neurol Neurol Sci Open Access. 2(1), 1008.
Publisher | Google Scholor - Piñeyro, G., & Blier, P. (1999). Autoregulation of serotonin neurons: role in antidepressant drug action. Pharmacological reviews, 51(3), 533-591.
Publisher | Google Scholor - Nakayama, K. (2002). Effect of paroxetine on extracellular serotonin and dopamine levels in the prefrontal cortex. Naunyn-Schmiedeberg's archives of pharmacology, 365, 102-105.
Publisher | Google Scholor - Hirano, K., Seki, T., Sakai, N., Kato, Y., Hashimoto, H., Uchida, S., & Yamada, S. (2005). Effects of continuous administration of paroxetine on ligand binding site and expression of serotonin transporter protein in mouse brain. Brain research, 1053(1-2), 154-161.
Publisher | Google Scholor - Kopschina Feltes, P., Doorduin, J., Klein, H. C., Juárez-Orozco, L. E., Dierckx, R. A., Moriguchi-Jeckel, C. M., & de Vries, E. F. (2017). Anti-inflammatory treatment for major depressive disorder: implications for patients with an elevated immune profile and non-responders to standard antidepressant therapy. Journal of Psychopharmacology, 31(9), 1149-1165.
Publisher | Google Scholor - Roman, M., & Irwin, M. R. (2020). Novel neuroimmunologic therapeutics in depression: a clinical perspective on what we know so far. Brain, behavior, and immunity, 83, 7-21.
Publisher | Google Scholor - Müller N., Schwarz M.J., Dehning S., Douhe A., Cerovecki A., Goldstein-Müller B., Spellmann I., Hetzel G., Maino K., Kleindienst N., et al. (2006). The cyclooxygenase-2 inhibitor celeCOXib has therapeutic effects in major depression: Results of a double blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol. Psychiatry. 11:680-684.
Publisher | Google Scholor - Akhondzadeh, S., Jafari, S., Raisi, F., Nasehi, A. A., Ghoreishi, A., Salehi, B., ... & Kamalipour, A. (2009). Clinical trial of adjunctive celecoxib treatment in patients with major depression: a double blind and placebo-controlled trial. Depression and anxiety, 26(7), 607-611.
Publisher | Google Scholor - Abbasi, S. H., Hosseini, F., Modabbernia, A., Ashrafi, M., & Akhondzadeh, S. (2012). Effect of celecoxib add-on treatment on symptoms and serum IL-6 concentrations in patients with major depressive disorder: randomized double-blind placebo-controlled study. Journal of affective disorders, 141(2-3), 308-314.
Publisher | Google Scholor - Na, K. S., Lee, K. J., Lee, J. S., Cho, Y. S., & Jung, H. Y. (2014). Efficacy of adjunctive celecoxib treatment for patients with major depressive disorder: a meta-analysis. Progress in Neuro-psychopharmacology and Biological Psychiatry, 48, 79-85.
Publisher | Google Scholor - Müller, N. (2019). COX-2 inhibitors, aspirin, and other potential anti-inflammatory treatments for psychiatric disorders. Frontiers in Psychiatry, 10, 375.
Publisher | Google Scholor - Trongtorsak, P., Olankijanunt, W., Trongtorsak, A., & Intamaso, U. (2018). Antidepressant and anti-inflammatory effects of a combined fluoxetine and celecoxib treatment in a rat model of depression. Chulalongkorn Medical Journal, 62(4), 653-665.
Publisher | Google Scholor - Singh, T., & Goel, R. K. (2016). Managing epilepsy-associated depression: Serotonin enhancers or serotonin producers? Epilepsy & Behavior: E&B, 66, 93-99.
Publisher | Google Scholor - Köhler, O., Petersen, L., Mors, O., & Gasse, C. (2015). Inflammation and depression: combined use of selective serotonin reuptake inhibitors and NSAIDs or paracetamol and psychiatric outcomes. Brain and behavior, 5(8), e00338.
Publisher | Google Scholor - Köhler, O., Benros, M. E., Nordentoft, M., Farkouh, M. E., Iyengar, R. L., Mors, O., & Krogh, J. (2014). Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA psychiatry, 71(12), 1381-1391.
Publisher | Google Scholor - Crespi F. (2010a) Wireless in vivo voltammetric measurements of neurotransmitters in freely behaving rats. Biosens. Bioelectr. 25, 2425-2430.
Publisher | Google Scholor - Crespi F. (2010b) Further Electrochemical and Behavioural Evidence of a Direct Relationship Between Central 5-HT and Cytoskeleton in the Control of Mood. The Open Neurol. J. 4, 5-14.
Publisher | Google Scholor - Crespi F. (2019) Concomitant in vivo voltammetric and electrophysiological analysis indicate that nociceptin/orphanin FQ may affect dopamine and then serotonin activities in the substancia nigra. Int. Physiol. J. 2(3), 1-9.
Publisher | Google Scholor