Case Report
Simultaneous Abrupt Coronary Artery Occlusion during Angiography: A Rare Case of Cardiac Arrest and Rescue
- Abbas Andishmand *
- Seyed Mostafa Seyedhosaini
Associate Professor of Cardiology and Interventionalist Yazd Cardiovascular Research Center, Shahid Sadoughi University of Medical Sciences, Afshar Hospital, Jomhoori avenue, Yazd, Iran.
*Corresponding Author: Abbas Andishmand, Associate Professor of Cardiology and Interventionalist Yazd Cardiovascular Research Center, Shahid Sadoughi University of Medical Sciences, Afshar Hospital, Jomhoori avenue, Yazd, Iran.
Citation: Andishmand. A, Seyedhosaini. S.M. (2024). Simultaneous Abrupt Coronary Artery Occlusion during Angiography: A Rare Case of Cardiac Arrest and Rescue. Clinical Case Reports and Studies, BioRes Scientia Publishers. 7(2):1-10. DOI: 10.59657/2837-2565.brs.24.181
Copyright: © 2024 Abbas Andishmand, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: August 24, 2024 | Accepted: September 21, 2024 | Published: October 10, 2024
Abstract
This report describes a rare case of simultaneous coronary artery thrombosis during coronary angiography in a 44-year-old female patient with non-ST elevation myocardial infarction (NSTEMI). The patient experienced cardiac arrest but was successfully resuscitated. The case highlights the importance of recognizing and managing complications during angiography.
Keywords: coronary artery thrombosis; angiography; cardiac arrest; non-ST elevation myocardial infarction (NSTEMI); resuscitation
The learning points of this case report as follows
Recognizing Rare Complications: Simultaneous coronary artery thrombosis during angiography is a rare complication but can have devastating consequences. This case emphasizes the importance of being aware of such uncommon occurrences and being prepared to act swiftly. Prompt Recognition and Intervention: Early recognition of the complication is crucial for successful management. This case highlights the significance of promptly identifying the thrombotic occlusion and initiating appropriate interventions to restore blood flow. Multidisciplinary Approach: The management of simultaneous coronary artery thrombosis requires a multidisciplinary approach involving various healthcare professionals, including interventional cardiologists, cardiac surgeons, anesthesiologists, and critical care specialists. Collaboration among these experts is essential for optimal patient outcomes Treatment Strategies: The case report demonstrates the effectiveness of treatment strategies such as balloon angioplasty, thrombosuction, and coronary artery stenting in restoring blood flow. Additionally, supportive measures like inotropic drugs, temporary pacemakers, and respiratory support can play a vital role in stabilizing the patient's hemodynamic status.
Introduction
Simultaneous coronary artery thrombosis during angiography is an exceptionally rare event that can have devastating consequences [1]. Previous reports have primarily focused on patients diagnosed with ST-segment elevation myocardial infarction (STEMI) [2,3]. In this case report, we present the case of a 44-year-old female patient diagnosed with non-ST elevation myocardial infarction (NSTEMI) who experienced this uncommon complication during coronary angiography, resulting in cardiac arrest. The objective of this report is to highlight the unique challenges associated with simultaneous coronary artery thrombosis and emphasize the critical importance of early recognition and appropriate management for achieving favourable outcomes.
Case presentation
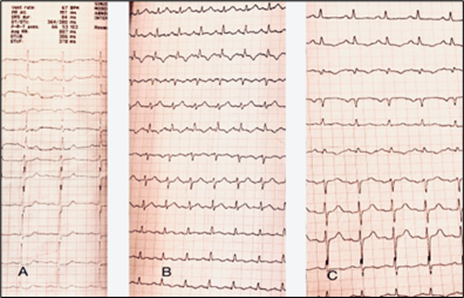
A 44-year-old female patient presented to our institution for evaluation of acute coronary syndrome. She had experienced typical chest pain and had been hospitalized two weeks prior to her visit to our center. Initial treatments for acute coronary syndrome were initiated, and she was prescribed daily doses of anti-platelet medications, including 81 mg of aspirin, 75 mg of clopidogrel, and 40 mg of atorvastatin. The patient had chronic microcytic anemia and no known cardiac disease and did not report any risk factors for coronary artery disease. On admission, her vital signs were stable, with normal cardiac auscultation, blood pressure of 125/80 mm Hg, a regular heart rate of 74 beats per minute, non-distended jugular veins, clear lungs, no peripheral edema, and a BMI of 26.3 kg/m2. The electrocardiogram showed ST depression changes in the anterior leads [Fig 1A]. Biomarker assays revealed a troponin level of 16000 ng/ml, while renal function tests showed normal results. The patient was diagnosed with non-ST elevation myocardial infarction (NSTEMI) and considered a suitable candidate for coronary angiography.
During the procedure, the initial left coronary injection revealed a narrowing of the proximal left anterior descending (LAD) artery (Figure 2A). Unexpectedly, the Judkins diagnostic catheter disengaged spontaneously and could not be reengaged. While attempting to replace the catheter with a smaller curve, the patient experienced convulsions accompanied by hypotension and bradycardia. Immediate intravenous administration of 1 mg atropine was performed, but the patient subsequently lost consciousness. This unexpected event rapidly worsened into cardiac arrest, necessitating immediate resuscitation measures. Cardiopulmonary resuscitation (CPR) was promptly initiated, following advanced cardiac life support (ACLS) protocols. The cardiac rhythm exhibited sinus bradycardia with a rate of 40 beats per minute. Due to the critical nature of the case and the compromised hemodynamic status of the patient, hemodynamic support measures were implemented, including the administration of inotropic drugs such as norepinephrine infusion to optimize cardiac contractility and blood pressure. Furthermore, a transvenous temporary pacemaker was inserted.
Figure 1: The electrocardiogram (ECG) of the patient, depicting ST-T changes at different time points. A) The baseline ECG obtained prior to the procedure exhibits ST segment depression in leads V3-V6. B) The ECG obtained after the procedure reveals mild ST elevation and T wave inversion in the inferolateral leads. C) The ECG on the day following the procedure demonstrates the amelioration of ST-T changes during the recovery period.
To diagnose the underlying causes of this unforeseen event and enable emergent interventions, a 6F left Judkins guiding catheter was carefully positioned at the opening of the left coronary artery under fluoroscopic guidance. Contrast injection confirmed complete occlusion of the proximal LAD artery (Figure 2A). Subsequently, a soft guidewire (Balancium, 0.014, Lepue Medical Technology Co., Ltd, China) was successfully advanced into the LAD artery to traverse the obstructed segment. The guidewire was meticulously manipulated and positioned beyond the obstruction, allowing for distal access. Following the successful positioning of the guidewire, balloon angioplasty was performed. An appropriately sized balloon catheter (Willma, 2*17; Sahajanand Medical Technologies Ltd.) was advanced over the guidewire and positioned across the lesion site. The balloon was then inflated, exerting pressure on the obstructive thrombosis and underlying plaque, effectively dilating the artery. Thrombosuction was also performed using an export catheter (Asp.cath, TSUNA MED).
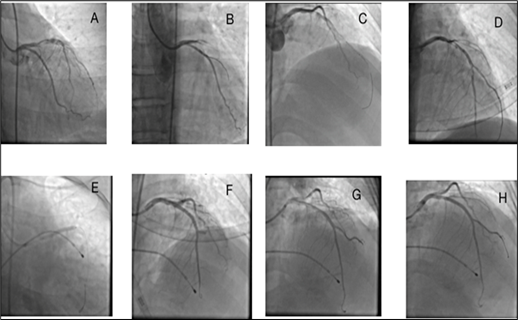
Throughout the procedure, careful monitoring of hemodynamic parameters and electrocardiographic changes was conducted to ensure patient safety. To further optimize the success of the intervention, intracoronary thrombolysis was initiated by administering reteplase 9 mg and eptifibatide (180 mcg/kg), in addition to an injection of 80 units/kg of unfractionated heparin. After several minutes of intense resuscitative efforts, a return of spontaneous circulation (ROSC) was achieved. Subsequent coronary angiography revealed successful restoration of blood flow in the LAD following thrombolytic therapy (Figure 2.B-D). To further enhance coronary patency and minimize the risk of recurrent thrombotic events, percutaneous coronary intervention (PCI) with stent placement was performed using a Firehawk stent (3*29; Microport) (Figure 2E).
Figure 2: Left coronary angiogram of the patient during diagnostic and intervention procedures on the left anterior descending artery (LAD) at A) First left coronary injection shows proximal significant stenosis of LAD. B) The second left coronary angiogram shows complete occlusion of LAD at the proximal segment. C) crossing of a soft wire across the occlusion. D)restoration of blood flow after intracoronary injections of reteplase and eptifibatide and thrombosuction and balloon angioplasty of the proximal lesion. E) stenting of the proximal lesion. F) angiogram after stenting shows clot embolization to the distal of LAD and also the diagonal branch. G) angiogram after balloon angioplasty of the distal part of LAD and diagonal branch shows restoration of blood flow to distal beds and H) final angiogram of the left coronary artery shows normal blood flow of all coronary segments.
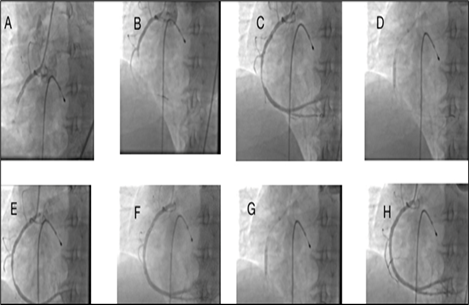
After the successful percutaneous coronary intervention (PCI) of the left anterior descending (LAD) artery, achieving TIMI flow 3 (Fig 2. F-H), the decision was made to proceed with the right coronary angiography to complete the evaluation of the coronary arteries. During the procedure, a proximal thrombotic obstruction was observed in the right coronary artery (RCA) (Figure 3.A). The right Judkins 6F catheter was immediately engaged to guide the intervention. Similar to the left coronary artery, the treatment measures consisted of balloon angioplasty and thrombosuction. The Right Judkins 6F catheter was skillfully advanced into the RCA, successfully crossing the obstructed segment with a guidewire, followed by thrombosuction to restore blood flow (Figure 3.B-C). Balloon angioplasty and stenting (Firehawk, 3.5*29; Microport) were performed, resulting in TIMI flow 3 in the RCA, indicating successful reperfusion (Figure 3.D-H). Notably, intravenous infusion of eptifibatide (2 mcg/kg/min) was initiated to prevent further platelet aggregation and thrombus formation. Respiratory support was provided by establishing an airway and initiating manual ventilation (Ambu bag).
Throughout the procedure and subsequent support measures, close monitoring of the patient's vital signs, including blood pressure, heart rate, and oxygen saturation, was performed. Gradually, the patient's systolic blood pressure improved from 20 mmHg at the beginning of the crisis to 100 mmHg at the end of the crisis. Furthermore, the patient regained consciousness, indicating a positive response to the interventions. Following the procedure, the patient was closely monitored in the cardiac intensive care unit (ICU), and her clinical condition gradually improved. She received appropriate medical therapy, including dual antiplatelet therapy, statins, and beta-blockers. The post-intervention electrocardiogram showed mild ischemic changes, and serial troponin assays revealed an increase from a baseline of 16000 ng/ml to 20000 ng/ml on the day after the event.
Figure 3: Right coronary angiogram of the patient during diagnostic and intervention procedures on right coronary artery (RCA) at A) First right coronary injection shows proximal cut off of RCA.B) angiogram shows crossing of a soft wire across the occlusion and position of the the wire in the RV branch. C) repositioning of the wire and crossing of a soft wire across the occlusion.D,E) restoration of blood flow after thrombosuction and balloon angioplasty. F) stenting of the proximal lesion, angiogram after stenting shows underexpntion of the stent. G) Balloon postdilation of the lesion for full expansion of stent and H) final angiogram of the right coronary artery shows normal perfusion.
Follow-up
After being discharged from the hospital, the patient was prescribed a medication regimen that included ticagrelor 90 mg twice daily, aspirin 81 mg daily, rosuvastatin 20 mg daily, and metoprolol 50 mg daily. Alongside pharmacological management, further diagnostic evaluations were conducted to investigate the possibility of a hypercoagulable state, collagen vascular disease, and COVID-19 infection. However, all diagnostic tests yielded normal results, suggesting that these factors did not contribute to the initial event.
During the one-month follow-up period, the patient remained asymptomatic and did not experience any complications. Echocardiography revealed normal left ventricular systolic function, with a left ventricular ejection fraction (LVEF) of 55% and a normal LV wall motion.
Discussion
The presence of simultaneous coronary artery thrombosis during angiography is a rare but potentially serious complication characterized by the formation of a thrombus in the coronary arteries, resulting in acute obstruction of blood flow. Rapid recognition and intervention are vital for successful management. This case report presents a patient who underwent coronary angiography but immediately developed a proximal thrombotic blockage in both the left and right coronary arteries. Urgent interventions, including balloon angioplasty, thrombosuction, and coronary artery stenting, were employed to restore blood flow. Supportive measures, such as inotropic drugs, temporary pacemaker placement, and respiratory support, were utilized to address the patient's compromised hemodynamic status. The patient responded positively to these interventions.
Simultaneous coronary artery thrombosis poses significant challenges and can have severe consequences, necessitating immediate intervention. Acute coronary artery occlusion, leading to severe ischemia and myocardial infarction, can occur due to various causes, including thrombosis, embolism, arterial dissection, and arterial spasm [5]. Prompt recognition and management are crucial in mitigating the effects of acute arterial occlusion. While thrombosis of an atherosclerotic lesion is widely recognized as the primary cause of acute coronary occlusion, it is important to consider alternative causes such as coronary dissection, embolism, and spasm. Although angiography can potentially lead to coronary occlusion through dissection or the embolism of air or debris into the coronary arteries, it is highly unlikely that bilateral thrombosis of the coronary arteries in our patient was caused by complications of the angiography catheter [6]. Table 1 provides an overview of different pathophysiological conditions, their clinical or predisposing factors, specific diagnostic tools used, and associated diagnostic findings [7-12].
Table 1: Pathophysiological mechanisms underlying coronary artery occlusion and diagnostic tools for differentiating between these mechanisms.
| Pathophysioloy | Clinical or Predisposing factors | Specific diagnostic tools | Diagnostic Findings |
| Atherosclerotic Plaque rupture and thrombosis | HTN, DM, HLP, smoking, familial CAD and Prior PCI or CABG or MI or PAD | CCTA, IVUS, OCT, CAG, clot aspiration | Intracoronary filling defects or haziness, thrombus extraction by aspiration catheter, visualization of thrombus by OCT or IVUS |
| Embolism (clot, air, tumor, foreign body) | Congenital heart disease, PFO, mechanical heart valve, VHD, cardiomyopathy, coronary catheterization or coronary intervention procedure, device implantation, Cardiac arrhythmias | Medical history, Physical examination, TTE, TEE, CMR, review of coronary angiogram, Ambulatory monitoring of rhythm, D-dimer test | Detection of right to left shunt, valvular heart disease, cardiomyopathy, visualization of clot, vegetation or tumor in the cardiac chambers or on the valves, severe LV systolic dysfunction, LV aneurysm, exit of clot with flushing of the diagnostic or guiding catheters, Simultaneous occurrence of CVA and MI, Absence of plaque on the site of coronary occlusion, Abrupt termination of the clot with a clear demarcation between the affected and unaffected arterial segments, and autopsy. |
| Vascular Spasm | Coronary intervention, coronary catheterization, Smoking, history of migraine, use of stimulant drugs such as cocaine or amphetamines, emotional stress, underlying coronary artery disease and endothelial dysfunction, | Review of medication and substance use, blood or urine levels of drugs or substances | ynamic ST elevation, improvement of stenosis or obstruction by intracoronary injection of nitroglycerin or verapamil or diltiazem, Provocative response to hyperventilation or intracoronary injection of acetylcholine or ergonovine |
| Dissection | female, Marfan syndrome, Ehlers-Danlos syndrome, fibromuscular dysplasia (FMD), arterial hypertension, pregnancy and peripartum period, familial dissection | CCTA, IVUS, OCT, CAG, MRI | Coronary Angiography: Radiolucent (dark) flap, Double lumen,Contrast staining Intravascular Ultrasound (IVUS) or Optical Coherence Tomography (OCT): Intimal flap, Disrupted arterial layers , Intramural hematoma Cardiac Magnetic resonance : intramural hematoma, Wall thickening and enhancement |
| Thrombophilias Inherited of Acquired | Age of less than 50 years, Family history of venous or arterial thrombosis, recurrent thromboembolic events, recurrent pregnancy loss, thrombus in unusual site, unprovoked thrombosis | Antiphospholipid tests, D-dimer test, specific genetic tests for factor V Leiden and prothrombin gene Prolonged activated partial thromboplastin time (aPTT) or prothrombin time (PT), doppler ultrasound study, CTA, MRI, V/Q scan | Prolonged aPTT or PT may indicate deficiencies in coagulation factors, such as protein C, protein S, or antithrombin,Detection lupus anticoagulant, anticardiolipin antibodies, or anti-beta-2 glycoprotein I antibodies, is associated with antiphospholipid syndrome (APS) and a hypercoagulable state, Genetic tests identify specific mutations associated with hypercoagulable conditions, such as factor V Leiden mutation or prothrombin gene mutation, Elevated levels of D-dimer suggest increased clot formation and breakdown ,Doppler ultrasound can assess the presence of deep vein thrombosis (DVT) in the lower extremities, suggesting a hypercoagulable state, Imaging modalities such as computed tomography (CT) angiography, magnetic resonance imaging (MRI), or ventilation-perfusion (V/Q) scan can help identify pulmonary embolism (PE) or arterial thrombosis |
| Systemic disease Autoimmune | History of Kawasaki disease, Systemic lupus erythematosus (SLE), Takayasu arteritis, Rheumatoid Arthritis (RA), Infections, Drug Reactions (such as minocycline, and some antiepileptic drugs), Behçet's Disease, giant cell arteritis and polyarteritis nodosa, Marfan syndrome and Ehlers-Danlos syndrome | Review of medical history and physical examination, CAG, CTA,MRI,complete blood count (CBC) , ESR orCRP , autoimmune markers,serological tests and biopsy | Coronary Angiography:stenosis or narrowing, aneurysms or dilatation Computed Tomography Angiography (CTA): stenosis, aneurysms Cardiac Magnetic Resonance Imaging (MRI): Myocardial edema,Late gadolinium enhancement Laboratory Tests: signs of inflammation,specific autoantibodies (e.g., antineutrophil cytoplasmic antibodies [ANCA] in ANCA-associated vasculitis), and serological tests to rule out infectious causes Biopsy: direct evidence of inflammation within the arterial walls. |
| Malignancy | Active malignancy, unusual/atypical presentation, evidence of hypercoagulability, chemotherapy agents, radiotherapy, and response to cancer treatment | CAG, CTA, Positron Emission Tomography (PET) Scan, Cardiac Magnetic Resonance Imaging (MRI), biopsy, tumor marker | Coronary angiography: evidence of tumor invasion or compression Histopathological examination:definitive evidence of tumor infiltration, presence of cancer cells within the arterial walls or surrounding tissues CT scans or MRI: direct evidence of tumor invasion into the coronary arteries or compression of the vessels |
| Infection | Recent infections such as Covid-19, vaccination, bacterial endocarditis, evidence of septic emboli and response to anti-microbial therapy | Blood culture, TTE, TEE, antibody titers, enzyme immunoassays (EIAs), polymerase chain reaction (PCR),cMR, | Elevated Inflammatory Markers: increased C-reactive protein (CRP) levels, erythrocyte sedimentation rate (ESR), or white blood cell count (WBC) Positive Blood Cultures: evidence of an active infection Echocardiography: valvular vegetations, valve dysfunction, myocardial abscesses Coronary Angiography: irregular narrowing or stenosis, intracoronary thrombi, or signs of vasculitis Histopathological Examination: reveal features such as inflammation, infiltration of inflammatory cells, or evidence of infectious agents within the vessel walls. |
The simultaneous occurrence of clot embolism in two non-adjacent territories is unlikely. On the other hand, coronary dissection can also lead to acute occlusion, but the presence of an intimal flap hinders the smooth passage of a guidewire and the substantial bulk of thrombus and distal embolization of the clot further support this diagnosis. One possibility is that the occlusion of the right coronary artery pre-existed, and the occlusion of the left coronary artery occurred during angiography. While this hypothesis cannot be definitively ruled out, there is no supporting evidence for it, such as ST changes in the lower leads or the presence of collateral coronary flow from the left to the right coronary artery. Therefore, acute simultaneous thrombosis remains the only leading cause of the alarming clinical manifestations observed in our patient., which was confirmed following successful guidewire advancement and a visible bulk of clots throughout of left and right coronary arteries. No similar reported cases have been found in the literature to date.
The incidence of simultaneous coronary artery thrombosis during angiography is relatively low, with reported rates varying in the literature. A literature search identified 29 articles reporting on 56 patients with this condition. The majority of patients were male, presented with cardiogenic shock, and had simultaneous thrombosis of the left anterior descending and right coronary arteries. Primary percutaneous coronary intervention (PCI) was the main treatment approach, and in-hospital mortality was 5%. Several risk factors have been identified, including advanced age, diabetes mellitus, hypertension, hyperlipidemia, smoking, previous history of coronary artery disease, complex lesion morphology, extensive catheter manipulation, and inadequate anticoagulation [13].
The exact mechanisms underlying simultaneous coronary artery thrombosis during angiography are not fully understood but likely involve vulnerable plaque disruption, platelet activation, and the procoagulant effects of the procedure itself. Clinical presentation typically manifests as acute coronary syndrome, and prompt recognition is crucial for timely intervention. Management requires a multidisciplinary approach, aiming to restore coronary blood flow and prevent thrombus propagation. Outcomes depend on the extent and duration of thrombotic occlusion, the promptness of intervention, and the overall clinical condition of the patient. Prevention strategies include careful patient selection, evaluation of risk factors, optimization of medical therapy, adherence to anticoagulation protocols, and the use of intracoronary imaging techniques.This case report highlights a unique instance of simultaneous thrombotic occlusion in a patient diagnosed with non-ST-elevation myocardial infarction during diagnostic angiography. Prompt and effective treatment interventions were successful in preserving the patient's life. Recognizing and promptly addressing such rare and life-threatening scenarios is essential for favorable patient outcomes. Further research and reporting of similar cases are warranted to enhance understanding and improve management strategies for this rare complication.
Conclusion
Simultaneous coronary artery thrombosis during angiography is a rare but potentially devastating complication. It is imperative to promptly recognize this condition, intervene early, and implement appropriate management strategies in order to achieve favorable outcomes for affected patients. Continued research and advancements in procedural techniques, peri-procedural monitoring, and pharmacological interventions are necessary to further enhance the prevention and management of this challenging complication.
Abbreviations
CAD: Coronary Artery Disease;
CAG: Coronary Angiography;
CBC: Complete Blood Count;
CHD: Congenital Heart Disease;
CMR: Cardiac Magnetic Resonance;
CVA: Cerebrovascular Accident;
DM: Diabetes Mellitus;
DVT: Deep Vein Thrombosis;
EIAs: Enzyme Immunoassays;
ESR: Erythrocyte Sedimentation Rate;
HTN: Hypertension;
HLP: Hyperlipidemia;
IVUS: Intravascular Ultrasound;
LV: Left Ventricular;
MI: Myocardial Infarction;
MRI: Magnetic Resonance Imaging;
OCT: Optical Coherence Tomography;
PFO: Patent Foramen Ovale;
PAD: Peripheral Arterial Disease;
PCR: Polymerase Chain Reaction;
PCI: Percutaneous Coronary Intervention;
PT: Prothrombin Time;
RF: Rheumatoid Factor;
TEE: Transesophageal Echocardiography;
TTE: Transthoracic Echocardiography;
VHD: Valvular Heart Disease;
V/Q: Ventilation-Perfusion Scan
Declarations
Acknowledgments
We would like to express our gratitude to the patient and her family for their consent and cooperation in sharing this case. We also acknowledge the dedicated efforts of the healthcare professionals involved in the resuscitation and subsequent management of the patient.
Conflict of Interest
The authors declare no conflicts of interest.
Ethics Approval
Ethics approval was obtained from the Institutional Review Board (IRB) of Shahid Sadoughi Medical School for the publication of this case report. Written informed consent was obtained from the patient for publication of this case report, including accompanying images
Funding
No specific funding was received for this case report
References
- Kim S, Seol SH, Park DH, Song YS, Kim DK, Kim KH, Kim DI, Song PS. (2018). Simultaneous multiple coronary arteries thrombosis in patients with STEMI. J Geriatr Cardiol, 15(3):241-243.
Publisher | Google Scholor - Pollak PM, Parikh SV, Kizilgul M, Keeley EC. (2009). Multiple culprit arteries in patients with ST segment elevation myocardial infarction referred for primary percutaneous coronary intervention. Am J Cardiol, 104(5):619-623.
Publisher | Google Scholor - Al Suwaidi J, Al-Qahtani A. (2012). Multiple coronary artery thrombosis in a 41-year-old male patient presenting with ST-segment elevation myocardial infarction. J Invasive Cardiol, 24(3):E43-E46.
Publisher | Google Scholor - May JE, Moll S. (2021). Unexplained arterial thrombosis: approach to diagnosis and treatment. Hematology Am Soc Hematol Educ Program, 2021(1):76-84.
Publisher | Google Scholor - Akbar H, Foth C, Kahloon RA, et al. Acute ST-Elevation Myocardial Infarction. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
Publisher | Google Scholor - Panduranga P, Riyami AA. (2010). Acute intracoronary thrombosis in a normal coronary artery following coronary angiography: thromboaspiration using a guide catheter. Heart Views. 11(2):68-70.
Publisher | Google Scholor - O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW. (2013). American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Jan 29;127(4):e362-425. doi: 10.1161/CIR.0b013e3182742cf6. Epub 2012 Dec 17. Erratum in: Circulation. 128(25):e481.
Publisher | Google Scholor - Stinton LM, Fritzler MJ. A clinical approach to autoantibody testing in systemic autoimmune rheumatic disorders. Autoimmun Rev. 2007 Nov;7(1):77-84.
Publisher | Google Scholor - Bachi K, Mani V, Jeyachandran D, Fayad ZA, Goldstein RZ, Alia-Klein N. Vascular disease in cocaine addiction. Atherosclerosis. 2017 Jul; 262:154-162.
Publisher | Google Scholor - Debbie Jiang MD, Alfred Ian Lee MD. (2019). Thrombotic Risk from Chemotherapy and Other Cancer Therapies. Cancer Treat Res, 179:87-101.
Publisher | Google Scholor - Cheruiyot I, Kipkorir V, Ngure B, Misiani M, Munguti J, Ogeng'o J. Arterial Thrombosis in Coronavirus Disease 2019 Patients: A Rapid Systematic Review. Ann Vasc Surg. 70:273-281.
Publisher | Google Scholor - Cines DB, Bussel JB. (2021). SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia. N Engl J Med. 384(23):2254-2256.
Publisher | Google Scholor - Mahmoud A, Saad M, Elgendy IY. (2015). Simultaneous multi-vessel coronary thrombosis in patients with ST-elevation myocardial infarction: a systematic review. Cardiovasc Revasc Med. 16(3):163-166.
Publisher | Google Scholor