Case report
Should Downstaging from Neoadjuvant Therapy Impact Selection of Adjuvant Osimertinib? -A Case Report
- Kathryn DeCarli 1*
- Jennifer Mingrino 2
- Maria Garcia-Moliner 2
- Paul Koffer 3
- Abbas E. Abbas 4
- Christopher G. Azzoli 5
1Department of Medicine, Division of Hematology/Oncology, Warren Alpert Medical School of Brown University, Providence, United States.
2Department of Pathology, Warren Alpert Medical School of Brown University, Providence, United States.
3Department of Radiation Oncology, Warren Alpert Medical School of Brown University, Providence, United States.
4Department of Thoracic Surgery, Warren Alpert Medical School of Brown University; Director of Thoracic Oncology, Lifespan Cancer Institute, Providence, United States.
5Associate Professor of Medicine, Warren Alpert Medical School of Brown University; Director of Thoracic Oncology, Lifespan Cancer Institute, Providence, United States.
*Corresponding Author: Kathryn DeCarli, Department of Medicine, Division of Hematology/Oncology, Warren Alpert Medical School of Brown University, Providence, United States.
Citation: K DeCarli, J Mingrino, Maria G-Moliner, P Koffer, A Abbas, et al. (2022). Should Downstaging from Neoadjuvant Therapy Impact Selection of Adjuvant Osimertinib? -A Case Report. International Journal of Medical Case Reports and Reviews, BRS Publishers. 1(1); DOI: 10.59657/2837-8172.brs.22.002
Copyright: © 2022 Kathryn DeCarli, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: October 24, 2022 | Accepted: November 15, 2022 | Published: November 21, 2022
Abstract
Existing data do not clearly define the role of Osimertinib in the treatment of resected EGFR-mutated lung cancer. We present the case of a patient with stage IIIA EGFR exon 19 deletion positive NSCLC whose disease was pathologically down staged by neoadjuvant chemoradiation. Future trials should include such patients to inform decisions about adjuvant treatment with respect to survival benefit and toxicity.
Keywords: resettable non-small cell lung cancer; Osimertinib; egfr; case report
Introduction
The ADAURA trial demonstrated that adjuvant Osimertinib prolongs disease-free survival in patients with resected, pathologic stage IB-IIIA lung cancers harboring an activating/sensitizing epidermal growth factor receptor (EGFR) mutation [1]. Patients who received neoadjuvant therapy were excluded from ADAURA, leaving the management of such patients open for debate. We present the case of a patient with stage IIIA/N2 lung adenocarcinoma with EGFR exon 19 deletion who was treated with neoadjuvant chemoradiation followed by lobectomy and was down staged by neoadjuvant treatment. This case highlights practical issues surrounding the timing of molecular pathology testing and the interpretation of pathologic response for selection of adjuvant therapy in contemporary practice.
Patient Information and Diagnostic Assessment
The patient is a 77 year old woman never-smoker with a past medical history of hypertension who presented with upper respiratory symptoms. A timeline of her evaluation and pre-operative treatment is shown in Table 1.
| Days after initial presentation | Event |
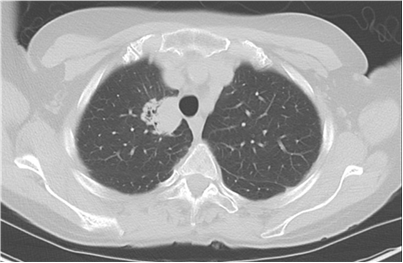
| Day 0 | Computed tomography (CT) of the chest obtained during evaluation of upper respiratory symptoms showed a 3.3cm right upper lobe mass with post obstructive atelectasis (Figure 1). By shared decision making, the patient was treated with antibiotics and plan for interval imaging which showed persistent mass. |
| Day 68 | Bronchoscopy with endobronchial ultrasound (EBUS) and fine needle aspiration (FNA) of right paratracheal (level 4) lymph nodes showed lung adenocarcinoma. Molecular pathology showed EGFR exon 19 deletion. |
| Day 81 | Pulmonary function tests showed >90% predicted values. |
| Day 90 | Magnetic resonance imaging (MRI) of the brain with intravenous (IV) gadolinium contrast was negative for metastases. |
| Day 97 | Positron emission tomography (PET)/CT confirmed fluorodeoxyglucose (FDG)-avid right upper lobe mass and ipsilateral hilar nodes. Based on abutting of the mediastinum and trachea, mediastinal involvement could not be excluded. |
| Days 119-162 | Neoadjuvant chemoradiation for clinical T3N2 NSCLC (stage IIIB). |
| Day 235 | Surgical resection. Date of surgery was delayed until after resolution of grade I anemia following completion of chemotherapy. |
Table 1: Timeline of the patient’s evaluation and pre-operative treatment.
Figure 1: CT scan at time of presentation showing 3.3 x 2.9cm right upper lobe mass.
Therapeutic Intervention
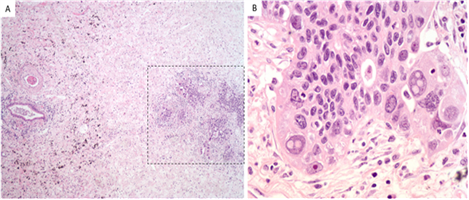
The patient was an excellent candidate for trimodality therapy given her PFTs, single station nodal involvement and low volume disease. The patient received 3 cycles of neoadjuvant carboplatin plus pemetrexed concurrent with chest radiation. She was offered intensity-modulated radiation therapy (IMRT) in definitive doses with 60 Gy in 30 fractions given concerns for potential resect ability with potential invasion into the mediastinum or trachea. She tolerated chemoradiation with grade 1 anaemia, anorexia, and fatigue. Repeat chest CT showed slight decrease in size of the mass. She then underwent robot-assisted thoracoscopy with right upper lobectomy and mediastinal lymph node dissection. Pathologic stage was ypT2aN0Mx (stage IB) based on visceral pleural invasion, with no residual cancer in lymph nodes, and only scant foci of residual adenocarcinoma on a background of extensive inflammatory changes (Figure 2). There was bronchopneumonia with abscess formation in the right upper lobe. Although not a pathologic complete response (CR), viable cancer cells involved only 5% of the tumour bed, consistent with a major pathologic response (MPR).
Figure 2: Histology from right upper lobectomy showing scattered microscopic foci of residual adenocarcinoma with surrounding fibrosis/elastosis, 40x (Figure 2a) and high-power image of irregular malignant glands, 400x, hematoxylin and eosin (Figure 2b).
In light of her clinical stage IIIA/N2 at diagnosis, and lack of medical contraindications, we decided to offer this patient adjuvant Osimertinib. She began Osimertinib 80mg daily. Unfortunately, 2 weeks later she died of an out-of-hospital cardiac arrest after an international flight. No post-mortem examination was performed.
Discussion
When planning combined-modality treatment for resettable IIIA/N2 NSCLC, the key clinical decision is whether to offer chemoradiation alone, chemoradiation followed by surgery, or chemotherapy followed by surgery. In contemporary practice, this decision is often independent of molecular pathology, or promise of benefit from consolidation or adjuvant drug therapy. Increasingly, molecular pathology testing is being used to select neoadjuvant treatment based on the results of phase 3 studies of neoadjuvant immune therapy which excluded patients with EGFR mutations or anaplastic lymphoma kinase (ALK) alterations [2].
Our case highlights the challenges of interpreting downstaging and MPR when deciding whether to offer adjuvant Osimertinib. MPR is correlated with survival outcomes in studies of neoadjuvant chemotherapy for resettable NSCLC [2, 3]. Phase II data support the use of MPR over radiographic response as a predictor of improved survival in patients who receive EGFR tyrosine kinase inhibitors (TKIs) prior to surgery. In a study demonstrating clinical activity of neoadjuvant gefitinib among patients with TKI-sensitive EGFR-mutated stage II-IIIA NSCLC, patients who had MPR had better survival than those who did not. Patients with radiographically stable disease achieved MPR at a similar rate as those with radiographic partial response [4]. Neoadjuvant erlotinib was shown to prolong median PFS compared with neoadjuvant chemotherapy in a randomized phase II study in which the rates of MPR were 9.7% for erlotinib vs 0% for chemotherapy [5].
In our case report, since the patient was treated with pre-operative radiation, the clinical importance of MPR is uncertain. Prospective trials of neoadjuvant chemoradiation have focused on nodal pathologic CR as the best predictor of survival outcomes. In a 1995 study of 126 patients with stage IIIA and IIIB NSCLC, negative mediastinal nodes at surgery were the strongest predictor of long-term survival after thoracotomy, and pathologic CR was not a significant predictor [6]. While potentially valuable for local control and hence cure, pre-operative radiation cofounds interpretation of response to neoadjuvant drug therapy.
This patient would not have qualified for the ADAURA study because she received neoadjuvant therapy. In ADAURA, patients with pathologic stage IB had less benefit, and better survival in the control arm, making the possibility of overtreatment highest in this subgroup.
The benefit of adjuvant Osimertinib appears to rely on a long duration of therapy because EGFR TKIs suppress cancer growth but do not eradicate the cancer. Any opportunity to eradicate disease with pre-operative therapy may be more valuable than adjuvant TKI therapy. In this case, there were some residual cancer cells in the resected tumour specimen. It is interesting to consider that we would have offered adjuvant Osimertinib to this patient based on her original clinical stage even if she had a pathologic CR to neoadjuvant chemoradiation.
Unless contemporary clinical trials are designed to de-escalate treatment based on response to neoadjuvant therapy, the evidentiary base will prompt physicians to choose the most aggressive local treatment, combined with the most expansive systemic treatment for their patients. With more routine pre-operative drug therapies anticipated, we predict a role for universal upfront molecular pathology testing for resettable NSCLC. Current neoadjuvant drug trials can detect neoadjuvant efficacy (using surrogates for survival, such as MPR), but are not designed to measure adjuvant efficacy. Assigning patients to adjuvant therapy based on neoadjuvant drug response or detection of circulating tumour DNA may elucidate ways to de-escalate adjuvant drug delivery and spare patient’s overtreatment.
Conclusion
Existing data do not inform selection of adjuvant Osimertinib for patients with neoadjuvantly-treated resettable lung cancers. This case highlights how the evidentiary base prompts physicians to choose the most aggressive local treatment, combined with the most expansive systemic treatment for their patients. Contemporary clinical trials should be designed to de-escalate adjuvant therapy to refine adjuvant drug selection and minimize overtreatment. Although it improves pathologic response, pre-operative radiation therapy may fall out of favour if it interferes with response-based adjuvant treatment selection.
Declarations
Disclosure Statement of Conflict of Interest
The authors have no conflicts of interest to report. All authors made substantial contributions to drafting, revising, and finalizing this case report.
Informed Consent
The patient provided verbal informed consent for her de-identified health information to appear in this case report.
Funding Source
None.
References
- Wu YL, Tsuboi M, He J, et al. (2020). ADAURA Investigators. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med. 383(18):1711-1723.
Publisher | Google Scholor - Forde PM, Spicer J, Lu S, et al. (2022). Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med. 386(21):1973-1985.
Publisher | Google Scholor - Hellmann MD, Chaft JE, William WN Jr, Rusch V, Pisters KM, Kalhor N, Pataer A, Travis WD, Swisher SG, Kris MG. (2014). University of Texas MD Anderson Lung Cancer Collaborative Group. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol. 15(1):42-50.
Publisher | Google Scholor - Zhang Y, Fu F, Hu H,Wang S, Li Y, Hu H, et al. (2021). Gefitinib as neoadjuvant therapy for resectable stage II-IIIA non–small cell lung cancer: a phase II study. J Thorac Cardiovasc Surg. 161:434-442.
Publisher | Google Scholor - Zhong WZ, Chen KN, Chen C, Gu CD,Wang J, Yang XN, et al. (2019). Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of stage IIIA-N2 EGFRmutant non–small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol. 37:2235-2245.
Publisher | Google Scholor - Albain KS, Rusch VW, Crowley JJ, et al. (1995). Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 13:1880-1892.
Publisher | Google Scholor