Review Article
Serum Malondialdehyde (MDA) Levels as A Biomarker of Covid-19-A Diagnosis, Surveillance and Preventive Measure
- Dr. Mohammad Nadeem Khan Ph.D *
- Dr. Ashok D. Kumar M.D
Department of Pharmacology, Clinical Pharmacology, Sri Aurobindo University, Indore, Madhya Pradesh, India.
*Corresponding Author: Dr. Mohammad Nadeem Khan, Department of Pharmacology, Clinical Pharmacology, Sri Aurobindo University, Indore, Madhya Pradesh, India.
Citation: Mohammad N. Khan, Ashok D. Kumar. (2024). Serum Malondialdehyde (MDA) Levels as A Biomarker of Covid-19-A Diagnosis, Surveillance and Preventive Measure. Journal of BioMed Research and Reports, Biores Scientia Publishers. 4(5):1-12. DOI: 10.59657/2837-4681.brs.24.070
Copyright: © 2024 Mohammad Nadeem Khan, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: January 23, 2024 | Accepted: February 15, 2024 | Published: February 24, 2024
Abstract
The last two to three years were quite frightening for the medical health sector. In this time interval, the whole human civilization was facing serious problems pandemic outbreaks in all over the globe. Due to covid-19 no medicine was working in this pandemic. In view of the potential threat of pandemic, scientists around the world have been running to understand SARS-CoV-2 and discover the screening methods of this disease to find out potential screening, surveillance and prevention techniques invented. In this review article we discuss new approach to screening and monitoring the covid-19 infection. Advances in medical biosciences technology provide new methods for novel virus detection, quantify and severity. Analytical tools like spectroscopic /HPLC techniques for MDA molecular markers estimation offer a valuable alternative to covid-19 diagnosis and evaluation studies, management. The different approach to standardization of MDA estimation system which have been developed need to be field tested and the field data is collected so that the complete technology packages could be ready for commercialization and transfer to the user agencies.
Keywords: severe acute respiratory syndrome (SARS); Covid-19; MDA; lipid per-oxidation; ROS; oxidative stress; communicable diseases; screening
Introduction: Overview of COVID-19
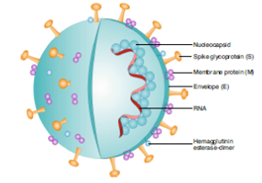
COVID-19 is a worldwide, zoonotic disease caused by the novel coronavirus, a member of the Coronaviridae family. Coronaviruses are enveloped viruses with non-segmented, single-stranded, positive-sense RNA genomes [1]. There are six known human coronaviruses, with severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) being highly pathogenic. Coronaviruses are classified under the order Nidovirales, family Coronaviridae, and subfamily Coronavirinae. Coronavirinae is divided into four genera: α-coronavirus, β-coronavirus, Gama-coronavirus, and Delta-coronavirus [2]. Beta-coronavirus has four distinct lineages (A, B, C, D), and SARS-CoV and MERS-CoV belong to lineage B. Coronaviruses have pleomorphic or spherical structures with an average diameter of 80–120nm [3]. The virion surface is decorated with club-like projections made up of the trimeric spike (S) glycoprotein. Some beta coronaviruses (e.g., HCoV-OC43 and HCoV-HKU1) have dimeric projections made up of the hemagglutinin-esterase (HE) protein. Other structural proteins include the membrane (M) glycoprotein, envelope (E) protein, and nucleo-capsid (N) protein (Figure-1) [4].
Figure 1: Schematic representation of SARS-CoV-2 structure. This is an enveloped, positive-sense RNA virus with four main structural proteins, including spike (S) and membrane (M) glycoproteins, as well as envelope (E) and nucleocapsid (N) proteins
Diagnostic methods include parasitological, radiological, and histo-pathological studies, as well as serological tests. Screening and therapeutic approaches for COVID-19 face challenges due to the novelty of the disease, and effective drugs and vaccines are still unavailable [5]. Collection of proper respiratory tract specimens at the right time and from the correct anatomical site is crucial for accurate molecular diagnosis. Real-time RT-PCR assays are the preferred molecular tests for diagnosing SARS-CoV-2 infections. Antigen-antibody-based techniques are being introduced as supplemental tools [6]. Current screening/diagnosis techniques have limitations, and there is a need for alternative tools that are easy to handle, cost-effective, and highly accurate [7]. Random access, integrated devices available at the point of care with scalable capacities are proposed to facilitate rapid and accurate screening and monitoring of SARS-CoV-2 infections [8]. This covers recent issues and challenges for the laboratory screening/diagnosis of infections caused by SARS-CoV-2. In the pre -analytical stage, collecting the proper respiratory tract specimen at the right time from the right anatomic site is essential for a prompt and accurate molecular diagnosis of COVID-19. Appropriate safety and efficacy measures are compliance to keep laboratory staff safe while producing rational test results. In the analytic stage, real -time RT -PCR assays remain the molecular test of choice for the etiologic diagnosis of SARS-CoV-2 infection while antigen-antibody based techniques are being introduced as supplemental tools. But all present screening/diagnosis techniques have a limitations and risk for negative, false positive result accuracy. In based upon above limitation we looking for other screening tools which have easy to handling, cost-effective and high accuracy potentials [9]. Therefore, currently proposed techniques, random access, integrated devices available at the point of care with scalable capacities will facilitate the rapid and accurate screening and monitoring of newly SARS-CoV-2 infections and greatly assist in the control of this outbreak.
Recently diagnosis challenges
Recommends using Nasopharyngeal (NP) swabs for early diagnosis or screening due to higher diagnostic yields, better patient tolerance, and increased operator safety. Suggests combining NP and Oropharyngeal (OP) swabs for increased sensitivity but acknowledges the need for twice the number of swabs [10]. Proposes alternatives like self-collected saliva or nasal washes for epidemiological screening, especially for asymptomatic individuals ("worried well"). Advocates reserving NP swabs for hospitalized patients, with the possibility of using deep sputum or Broncho-alveolar Lavage (BAL) samples for those who initially test negative [11]. Highlights the importance of understanding the need for repeated testing or the use of bronchoscopy in severe cases if the initial screening test is negative. Calls attention to the insufficiently studied role of rectal swabs in advanced infections or as a test of infectivity/cure, stressing the urgency of further research in this area [12]. Stresses the need for broad screening/testing, involving both molecular and serological testing, to accurately estimate the true mortality rate and other epidemiological markers. Quick Development of Point-of-Care Molecular Devices: Emphasizes the critical importance of developing integrated, random access, point-of-care molecular devices for the accurate screening of COVID-19 infections. Describes these rapid STAT tests as significant for real-time patient management and infection control decisions, especially in situations where resources for respiratory isolation are limited. In summary, the commentary underscores the importance of optimizing screening methods, resource allocation, and the development of rapid diagnostic tools for effective and efficient management of the COVID-19 pandemic [13]. The emphasis on understanding the dynamics of testing, exploring alternative specimens, and broadening testing approaches contributes to a comprehensive strategy in addressing the challenges posed by the virus [14].
Overview of MDA
The role of super oxygen radicals (SOR) or Reactive Oxygen Species (ROS) in biological systems, particularly in the context of parasitic infections [15]. It also introduces the concept of oxidative stress and its potential physiological and pathological impacts. Additionally, the study mentioned focuses on measuring the serum concentration of malondialdehyde (MDA), an end-product of lipid peroxidation, in humans with COVID-19 to understand its relevance in the pathological mechanism of the disease [16]. ROS, including superoxide radicals (O2), hydrogen peroxide (H2O2), and hydroxyl radicals (OH), are produced by aerobic organisms and can have both physiological and harmful effects [17]. ROS are highly reactive and can be toxic, mutagenic, or carcinogenic. Despite their potential harm, ROS at sub-toxic concentrations play crucial physiological roles in biological systems [18]. The passage mentions an active area of research exploring the role of highly reactive oxygen free radicals in the pathogenesis of parasitic infections. Polymorphonuclear neutrophils (PMNL) and monocyte/macrophage cells, part of the non-specific immune system, generate large amounts of toxic substances, including ROS and reactive nitrogen species (RNS) [19-20]. ROS and RNS, such as nitric oxide (NO), can have cytotoxic effects on bacteria, parasites, and tumor cells[21]. ROS and RNS have the capacity to degrade biomolecules, leading to oxidative stress [22,23,24]. Lipid peroxidation, involving the degradation of membrane lipids, can compromise cell integrity and function. The study mentioned in the passage measures the serum concentration of malondialdehyde (MDA), an indicator of lipid peroxidation, in humans with COVID-19 [25,26]. Increased levels of MDA are associated with lipid peroxidation and have been correlated with various chronic diseases, including parasitic infections [27,28,29]. The study aims to understand the potential involvement of oxidative stress, ROS, and lipid peroxidation in the pathological mechanisms of COVID-19 by assessing MDA levels in infected individuals.
Table 1: Main viruses associated with reactive oxygen species (ROS) production.
| Virus |
| Human immunodeficiency virus (HIV) |
| Hepatitis B virus (HBV) and hepatitis C virus (HCV) |
| Epstein-Barr virus (EBV) |
| Herpes simplex type 1 (HSV-1) and vesicular stomatitis virus (VSV) |
| Respiratory syncytial virus (RSV) |
| Influenza viruses |
| Human T cell leukaemia virus type 1 (HTLV-1) |
Biochemistry and generation of MDA In-vivo and In vitro
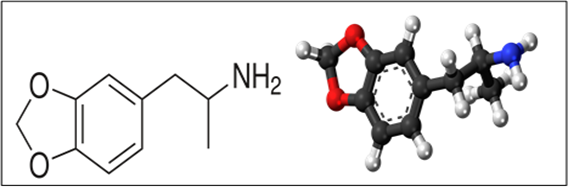
In-depth explanation of the generation and effects of reactive oxygen species (ROS) and focuses on the role of malondialdehyde (MDA) as a reactive terminal degradation product [30,31]. Cellular respiration in aerobic organisms produces energy, but it also generates ROS as by-products. ROS are produced endogenously in mitochondria, peroxisomes, the endoplasmic reticulum, and the plasma membrane. Exogenous sources include UV light, heat, bacterial agents, tobacco smoke, and ionizing radiation [32]. ROS can react with membrane lipids, particularly polyunsaturated fatty acids (PUFAs), and leading to lipid peroxidation. Lipid peroxidation, both enzymatic and non-enzymatic, can result in the perturbed integrity of cellular structures, potentially leading to cellular death. Enzymatic mechanisms involve the activation of lipoxygenases, myeloperoxidases, cyclooxygenases, and cytochrome P-450.Peroxydienyl radicals are generated, leading to the production of lipid hydroperoxide molecules (LOOH) [33,34,35]. Non-enzymatic mechanisms involve free radicals generated by NADPH oxidases and nitric oxide synthases [36]. Free radicals remove hydrogen from PUFAs, resulting in the generation of lipid-peroxides. Cellular antioxidant mechanisms involve enzymes such as glutathione peroxidases, superoxide dismutases, and catalases [37]. Antioxidants like Vitamin C and Vitamin E act non-enzymatically to counteract the effects of ROS. Imbalance between oxidative stress and antioxidant mechanisms can lead to pathological oxidation [38]. End products of lipid peroxidation, including malondialdehyde (MDA), are used as indicators of oxidative stress in higher eukaryotic organisms [39]. MDA is an aldehyde with the chemical formula C3H4O2 that mainly exists in its enol form at physiological pH7.6 (figure-2) [40,41]. Newly generated MDA can modify free amino acids, proteins, nucleotides, and phospholipids, creating stable covalent epitopes. Major carriers of MDA epitopes include apoptotic/necrotic cells, micro-vesicles (MV), and oxidized lipoprotein particles [42, 43]. Arachidonic acid (AA, 20:4) and docosahexaenoic acid (DHA, 22:6) in membrane phospholipids are substrates for MDA generation [44, 45]. Proposed mechanisms involve the formation of intermediates and breakdown through thermal or acid-catalyzed reactions. MDA can also be generated as a side product of thromboxane A2 synthesis and through the oxidation of spermine, UV-irradiation of skin surface lipid squalene, or gamma irradiation of carbohydrates. The detailed explanation provides insights into the intricate processes of ROS generation, lipid peroxidation, and the significance of MDA in cellular responses to oxidative stress [46].
Figure 2: Chemical structure of MDA(2D,3D)
Generation of MDA epitopes
The characteristics and reactivity of malondialdehyde (MDA), particularly in the context of its pH-dependent behavior and its potential to form immunogenic epitopes, such as malondialdehyde-acetaldehyde (MAA) [47]. Liberated MDA exists as a bi-functional electrophile, and its reactivity is influenced by pH levels. At physiological pH (7.6), MDA primarily exists in the enolate-ion form lowering the pH (acidic conditions) can increase MDA reactivity, favoring the formation of beta-hydroxyacrolein and enhancing its affinity towards nucleophilic molecules [48].
At high concentrations of MDA in an acidic milieu (pH range 4-7), long oligomers of MDA can form hydrolytic cleavage of these MDA oligomers generates MDA and acetaldehyde (AcA) [48]. Monitoring the formation of MDA oligomers is crucial, as AcA and MDA can react to create malondialdehyde-acetaldehyde (MAA) immunogenic epitopes. MAA epitopes are more complex forms with potential immunogenic properties. Free AcA and MDA possess the ability to create epitopes on major cellular macromolecules in different tissues [49]. They serve as mediators of oxidative stress due to their chemical nature. The formation of stable epitopes with biomolecules, suggested to increase the half-life of MDA in vivo, is highlighted. MDA modifications of lipids, nucleotides, free amino acids, and proteins may lead to functional loss, structural integrity compromise, and altered cellular responses [50]. The passage indicates that modifications of each type of macromolecule (lipids, nucleotides, free amino acids, and proteins) will be discussed separately, potentially providing a detailed exploration of the consequences of MDA-induced modifications [51]. This segment underscores the dynamic reactivity of MDA, its dependence on pH, and the potential implications of its interactions with acetaldehyde in forming immunogenic epitopes[52]. The consequences of MDA modifications on different macromolecules are highlighted as essential aspects of understanding its role in oxidative stress and cellular responses.
MDA epitopes in diseases
Diseases associated with increased levels of malondialdehyde (MDA) and highlights the role of MDA in various chronic and acute conditions related to oxidative stress [53]. Additionally, it mentions the involvement of highly reactive oxygen free radicals (ROS) in the pathogenesis of parasitic infections and viral diseases [54]. Cardiovascular diseases Neurodegenerative diseases Metabolic diseases Communicable diseases MDA levels in the plasma of healthy individuals show great variability, influenced in part by the method of blood drawing and sample preparation. Leishmania Plasmodium falciparum (malaria)Ascaris lumbricoides Toxoplasma gondii Trypanosoma cruzi [55]. Viral infections can trigger ROS production, either through cytokines secreted in response to the pathogen or by viral components [56]. Lipid peroxidation, a physiological process, is implicated in the pathogenesis (intensity of virulence) of various parasitic diseases [57]. ROS-induced lipid peroxidation disrupts cell membranes, leading to necrotic death. Infection with various parasites is associated with a marked elevation in lipid peroxidation [58]. The commonly used TBARS assay for MDA estimation is known to be non-specific. More reliable methods, such as MDA-specific antibodies or mass spectrometry-based assays, exist but their reliability as biomarkers for all diseases remains unclear [59]. The passage emphasizes the broad spectrum of diseases linked to increased MDA levels and highlights challenges in accurately estimating MDA, especially in healthy individuals and in the context of specific diseases [60]. It underlines the need for more specific and reliable biomarkers for assessing oxidative stress in various pathological conditions.
Estimation methods of MDA methods
Spectrophotometric estimation
Commonly used method for measuring lipid peroxidation as an indicator of reactive oxygen species (ROS)-mediated damage to cell membranes [61]. The method involves using a spectrophotometer at specific wavelengths or a spectrometer for detection. Malondialdehyde (MDA) is highlighted as one of the well-studied end-products of lipid peroxidation and is frequently used to estimate oxidative stress conditions in clinical samples [62]. Lipid peroxidation is commonly used as an indicator of ROS-mediated damage to cell membranes. Measurement is done using a spectrophotometer at an absorbance of 520-535nm or by a spectrometer at specific excitation and emission wavelengths (λ excitation = 515nm, λ emission =555nm) [63,64]. Malondialdehyde (MDA) is a well-studied end-product of peroxidation of polyunsaturated fatty acids [65]. The amount of MDA is frequently estimated using the thiobarbituric acid reactive substances (TBARS) technique. Various methods for MDA estimation include those described by Donnan [66], Yagi [67], Mihara and Uchiyama [68], Buege and Aust [69], Ohkawa et al. [70], Yoshioka et al [71]. In these methods, MDA reacts with TBARS in an acidic medium at 100°C to produce a pink/red-colored product [72]. MDA may also react with TBARS, producing derivatives that absorb light in the same wavelength range. A kit for MDA estimation is mentioned as being available, providing a convenient option for researchers and laboratories [73]. This passage outlines the practical aspects of measuring lipid peroxidation, particularly focusing on MDA estimation using the TBARS technique [74]. The described methods offer a fast, sensitive, and easy approach for assessing oxidative stress conditions in clinical samples.
Other alternative approach
The alternative methods for estimating blood plasma malondialdehyde (MDA) levels, along with other lipid peroxidation biomarkers. These methods offer different approaches with varying levels of reproducibility, reliability, and practicality [75]. HPLC using a C-18 reversed-phase column is mentioned for estimating blood plasma MDA levels. The method is described by Victorino et al. and provides reproducibility and reliability [76]. MDA estimation can also be done by GC-MS on a capillary column following trans-methylation with sodium methoxide. While more reproducible and reliable, this method is considered time-consuming, labor-intensive, and impractical for individual sample processing [77]. Various biomarkers, such as 8-isoprostaglandinF2α (8-iso-PGF2α), 4-hydroxy-2-nonenal (4-HNE), conjugated dienes (CD), and lipid hydroperoxides (LOOH), are mentioned.8-Iso-PGF2α, a result of non-enzymatic peroxidation of arachidonic acid, can be measured using rapid ultra-high-performance liquid chromatography-tandem mass spectrometry [78]. The method is noted for being labor-intensive and requiring specialized and expensive instrumentation. Unsaturated hydroxyalkenal 4-HNE can be evaluated in tissues, preferably using immunohistochemistry (IHC) or HPLC. CDs, resulting from free radical-induced autoxidation of polyunsaturated fatty acids (PUFAs), can be investigated based on the maximal absorption of UV light at 233nm. LOOHs, primary oxidation products of PUFAs, can be estimated by the ferrous oxidation xylenol-orange (FOX) assay. This assay is based on the ability of LOOH to oxidize ferrous iron in the presence of xylenol orange, forming a colored ferric-xylenol orange complex with absorbance at 560nm [79]. These alternative methods and biomarkers provide diverse options for researchers to assess lipid peroxidation and oxidative damage to cell membranes. Each method has its advantages and limitations, and the choice depends on the specific requirements of the study.
Application of Serum MDA Estimation for covid-19 pandemic
- Very easy error free sampling
- Human error is very less almost negligible at sample collection
- It will be mostly using randomized/surveillance testing in general population
- Easy operative and result interpretation techniques.
- Very helpful to knowing virus load in infected patient
- Easy to knowing severity of infection
- It will also enable also asymptomatic covid-19 infected individuals
- it will be very economical so easily used as whole population
- It will be helpful for therapeutic management of covid-19 patient
- It will also enable to measure immunity index of general population
- Reduce the risk of lab personals which handling the sample
- Testing cost approximately 25/test manual method, 55/kit method, 200/ hplc method.
- Testing time 1hour spectroscopic method and 3-hour hplc methods
- Less safety measure required for sampling transportation
- Not specific, extra training required to lab technicians
- It will be helpful to research also (for therapeutic research)
- Setup of surveillance lab easily as well as establish in very remote area
- Less lab facility required
- This testing techniques also helpful to assessing health index of population
- It will be applicable assessing healthy person immunity level
Limitations
- Standardization required
- Cross examination is measure issue
- Set the infection range value
- Increase the accuracy
Practical relevance
The challenges and cost matter of covid-19 infection diagnosis has made necessary the development of new user-friendly methods. Advances in medical biosciences technology provide new methods for novel virus detection, quantify and severity. Analytical tools like spectroscopic /HPLC techniques for MDA molecular markers estimation offer a valuable alternative to covid-19 diagnosis and evaluation studies, management. This research may summarize the alternate cost-effective techniques in plant COVID-19 pandemic investigation and surveillance with special accentuation on the screening and monitoring efforts of the MDA quantification.
Executive Summary
Novel corona virus pandemic is also central to innovative areas of current research scenario, including diagnosis and therapeutic management. The prime importance of covid-19 pandemic of surveillance/screening, entire population. Due to cost and sampling and facility challenges would be to required cost effective and simple handling utility techniques for covid-19 Diagnosis and evaluation. The significance of an efficient MDA estimation protocol would be too easy and cost-efficient techniques to screening maximum number of populations in minimum period of time with proper safety and accuracy along with therapeutic index against covid-19 pandemic. The different approach to standardization of MDA estimation system which have been developed need to be field tested and the field data is collected so that the complete technology packages could be ready for commercialization and transfer to the user agencies
References
- Huang, C. et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet.
Publisher | Google Scholor - Jiang, S., Du, L. & Shi, Z. (2020). An emerging coronavirus causing pneumonia outbreak inWuhan, China: calling for developing therapeutic and prophylactic strategies. Emerg. Microbes Infect, 9:275-277
Publisher | Google Scholor - Li, F., Li, W., Farzan, M. & Harrison, S. C. (2005). Structure of SARS coronavirus spikereceptor-binding domain complexed with receptor. Science, 309:1864-1868
Publisher | Google Scholor - Liu, S. et al. (2004). Interaction between heptad repeat 1 and 2 regions in spike protein ofSARS-associated coronavirus: implications for virus fusogenic mechanism andidentification of fusion inhibitors. Lancet, 363:938-947
Publisher | Google Scholor - Lu, L. et al. (2014). Structure-based discovery of Middle East respiratory syndrome coronavirusfusion inhibitor. Nat. Commun., 5:3067
Publisher | Google Scholor - Ravirala Venkateshwarlu et al. (2011). Phytochemistry and pharmacology of Alangium salvifolium: A review. Journal of Pharmacy Research, 4(5):1423-1425
Publisher | Google Scholor - Xia, S. et al. (2020). Fusion mechanism of 2019-nCoV and fusion inhibitorstargeting HR1 domain in spike protein. Cellular & Molecular Immunology.
Publisher | Google Scholor - Yuan, Y. et al. (2019). Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteinsreveal the dynamic receptor binding domains. Nat. Commun coronavirus spike. Sci. Adv. 5:eaav4580
Publisher | Google Scholor - Zhou, P. et al. (2020). A pneumonia outbreak associated with a new coronavirus ofprobable bat origin. Nature.
Publisher | Google Scholor - Bhaskar A , Bala J , Varshney A , Yadava P . (2011). Expression of measles virus nucleoprotein induces apoptosis and modulates diverse functional proteins in cultured mammalian cells. PLoS One, 6:e18765 .
Publisher | Google Scholor - Claus C, Sch ö nefeld K, H ü bner D, Chey S , Reibetanz U , Liebert UG . (2013). Activity increase inrespiratory chain complexes by rubella virus with marginal induction of oxidative stress. J Virol, 87:8481-8492.
Publisher | Google Scholor - Hosakote YM , Komaravelli N , Mautemps N , Liu T , Garofalo RP , Casola A. (2012). Antioxidant mimetics modulate oxidative stress and cellular signaling in airway epithelial cells infected with respiratory syncytial virus. Am J Physiol Lung Cell Mol Physiol., 303:L991-1000 .
Publisher | Google Scholor - Hosakote YM , Liu T , Castro SM , Garofalo RP , Casola A . (2009). Respiratory syncytial virus induces oxidative stress by modulating antioxidant enzymes. Am J Respir Cell Mol Biol, 41:348-357 .
Publisher | Google Scholor - Hosakote YM , Jantzi PD , Esham DL , Spratt H , Kurosky A , Casola A , Garofalo RP. (2011). Viral-mediated inhibition of antioxidant enzymes contributes to the pathogenesis of severe respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med, 183:1550-1560.
Publisher | Google Scholor - Agrawal L, Louboutin JP, Marusich E , Reyes BA , Van Bockstaele EJ , Strayer DS. (2010). Dopaminergic neurotoxicity of HIV-1 gp120: reactive oxygen species as signaling intermediates. Brain Res, 1306:116-130.
Publisher | Google Scholor - Harman D. (1981). The aging process. Proc Natl Acad Sci USA. 78(11):7124-7128.
Publisher | Google Scholor - Harman D. (1988). Free radicals in aging. Mol Cell Biochem, 84(2):155-161.
Publisher | Google Scholor - Klauning JE, Kamendulis LM. (2004). The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol.44:239-267.
Publisher | Google Scholor - Inal ME, Kanbak G, Sunal E. (2001). Antioxidant enzyme activities andmalondialdehyde levels related to aging. Clin Chim Acta., 305(1):75-80.
Publisher | Google Scholor - Ceballos-Picot I, Trivier JM, Nicole A, Sinet PM, Thevenin M. (1992). Agecorrelated modifications of copper–zinc superoxide dismutase andglutathione-related enzyme activities in human erythrocytes. ClinChem, 38(1):66-70.
Publisher | Google Scholor - Guemouri L, Artur Y, Herbeth B, Jeandel C, Cuny G, Siest G. (1991). Biologicalvariability of superoxide dismutase, glutathione peroxidase, and catalasein blood. Clin Chem, 1991;37(11):1932-1937.
Publisher | Google Scholor - Ahmed RG. (2005). Is there a balance between oxidative stress and antioxidantdefense system during development? Med J Islam World Acad Sci., 15(2):55-63.
Publisher | Google Scholor - Kurata M, Suzuki M, Agar NS. Antioxidant systems and erythrocyte lifespan in mammals. ComBiochem Physiol. 1993;106(3):477-487.
Publisher | Google Scholor - Hagihara M, Nishigaki I, Maseki M, Yagi K. (1984). Age-dependent changes inlipid peroxide levels in the lipoprotein fractions of human serum. J Gerontol. 39(3):269-272.
Publisher | Google Scholor - Marnett LJ. (2000). Oxyradicals and DNA damage. Carcinogenesis., 21(3):361-370.
Publisher | Google Scholor - Shigenaga MK, Hagen TM, Ames BN. (1994). Oxidative damage and mitochondrial decay in aging. Proc NatlAcad Sci USA, 91(23):10771-10778.
Publisher | Google Scholor - Bland JS. (1995). Oxidants and Antioxidants in Clinical Medicine: Past, Presentand Future Potential. J Nutr Environ Med, 5(3):255-280.
Publisher | Google Scholor - Taridi NM, Yahaya MF, Teoh SL, Latiff AA, Wan Ngah WZ, Das S, et.al. (2011). Tocotrienol Rich Fraction (TRF) supplementation protects againstoxidative DNA damage and improves cognitive function in WistarRats. Clin Ter., 162(2):93-98.
Publisher | Google Scholor - Makpol S, Durani LW, Chua KH, Mohd Yusof YA, Wan Ngah WZ. (2011). Tocotrienol-Rich Fraction Prevents Cell Cycle Arrest and Elongates Telomere Length in Senescent Human Diploid Fibroblasts. J BiomedBiotechnol, 506171.
Publisher | Google Scholor - Pin KY, Luqman Chuah A, Abdull Rashih A, Mazura MP, Fadzureena J. et al. Antioxidant and anti-inflammatory activities of extractsof betel leaves (Piper betle) from solvents with different polarities. J TropFor Sci., 22(4):448-455.
Publisher | Google Scholor - Bhattacharya S, Banerjee D, Bauri AK, Chattopadhyay S, Bandyopadhyay SK. (2007). Healing property of the Piper betel phenol, allylpyrocatechol against indomethacin-induced stomach ulcerationand mechanism of action. World J Gastroenterol, 13(27):3705-3713.
Publisher | Google Scholor - Dasgupta N, Bratati D. Antioxidant activity of Piper betle L. leaf extractin vitro. Food Chem. 2004;88:219-224.
Publisher | Google Scholor - Choudhary D, Kale RK. (2002). Antioxidant and nontoxic properties of Piperbetle leaf extract: in vitro and in vivo studies. Phytother Res., 16(5):461-466.
Publisher | Google Scholor - Adachi H, Ishii N. (2000). Effects of tocotrienols on life span and proteincarbonylation in Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci. 55(6):280-285.
Publisher | Google Scholor - Kamat JP, Sarma HD, Devasagayam TPA, Nesaretnam K, Basiron Y. (1997). Tocotrienols from palm oil as effective inhibitors of protein oxidationand lipid peroxidation in rat liver microsomes. Mol Cell Biochem. 170(1-2):131-138.
Publisher | Google Scholor - Chin SF, Ibahim J, Makpol S, Abdul Hamid NA, Abdul Latiff A, ZakariaZ, et al. (2011). Tocotrienol Rich Fraction Supplementation Improved LipidProfile and Oxidative Status in Healthy Older Adults: A RandomizedControlled Study. Nutr Metab (Lond), 8(1):42.
Publisher | Google Scholor - Hasegawa T, Kimura Y, Hiromatsu K, Kobayashi N, Yamada A, Makino M, et al. (1997). Effect of hot water extract of Chlorella vulgaris on cytokine expression patterns in mice with murine acquired immune deficiency syndrome after infection with Listeria monocytogenes. Immuno pharmacology, 35(3):273-382.
Publisher | Google Scholor - Mohd Yusof YA, Hassan Basari J, Mukti NA, Sabuddin R, Muda AR, et al. (2011). Fatty acids composition of microalgae Chlorellavulgaris can be modulated by varying carbon dioxide concentration in outdoor culture. African J of Biotech. 2011;10(62):13536-135342.
Publisher | Google Scholor - Mohd Yusof YA, Md Saad S, Makpol S, Shamaan NA, Wan Ngah WZ. (2010). Hot water extract of Chlorella vulgaris induced DNA damage andapoptosis. Clinics., 65(12):1-7.
Publisher | Google Scholor - Suhaniza S, Nor Aripin S, Wan Zurinah WN, Yasmin Anum MY. (2006). Chemopreventive effect of Chlorella vulgaris in choline deficient dietand ethionine induced liver carcinogenesis in rats. Int J Cancer Res., 2(3):234-241.
Publisher | Google Scholor - Pin KY, Chuah TG, Choong TSY, Abdull Rashih A, Rasadah MA, LawsCL. (2007). Modelling of extraction kinetics of hydroxychavicol from betel (Piperbetle L.) leaves. World Engineering Congress, Penang Malaysia.
Publisher | Google Scholor - Noor Aini AH, Illyana I, Wan Zurinah WN, Gapor MT, Musalmah M. (2003). Effect of Long Term Palm Oil Vitamin E Supplementation on Rate ofWound Closure and Lipid Peroxidation at Different Age Groups in Rats. Oxidants and Antioxidants in Biology. Cadiz, Spain.
Publisher | Google Scholor - Beyer WF Jr, Fridovich I. (1987). Assaying for superoxide dismutase activity:some large consequences of minor changes in conditions. Anal Biochem, 161(2):559-566.
Publisher | Google Scholor - Paglia DE, Valentine WN. (1967). Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Clin Med. 1967;70(1):158-169.
Publisher | Google Scholor - Aebi H. (1984). Catalase in vitro. Methods Enzymol., 105:121-126.
Publisher | Google Scholor - Pilz J, Meineke I, Gleiter CH. (2000). Measurement of free and boundmalondialdehyde in plasma by high-performance liquid chromatography as the 2,4-dinitrophenylhydrazine derivative. J Chromatogr BBiomed Sci Appl. 742(2):315-325.
Publisher | Google Scholor - Sim SA, Salonikas C, Naidoo D, Wilcken DEL. (2003). Improved method forplasma malondialdehyde measurement by high-performance liquid chromatography using methyl malondialdehyde as an internal standard. J Chromatogr B Analyt Technol Biomed Life Sci. 2003 785(2):337-344.
Publisher | Google Scholor - Waris G, Ahsan H. (2006). Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog, 5:14.
Publisher | Google Scholor - Moreira PI, Duarte AI, Santos MS, Rego AC, Oliveira CR. (2009). An integrativeview of the role of oxidative stress, mitochondria and insulin in Alzheimer’s disease. J Alzheimers Dis., 16(4):741-746.
Publisher | Google Scholor - Norfaizatul SO, Zetty Akmal CZ, Noralisa AK, Then SM, Wan ZurinahWN. (2010). Dual Effects of Plant Antioxidants on Neuron CellViability. J Med Plants, 9(6).
Publisher | Google Scholor - Arambewela L, Arawwawala M, Rajapaksa D. (2006). Piper betle: a potentialnatural antioxidant. Int J Food Sci Technol, 41(1):10-14.
Publisher | Google Scholor - Andersen HR, Nielsen YB, Nielsen F, Grandjean P. (1997). Antioxidative enzyme activities in human erythrocytes. Clin Chem., 43(4):562-528.
Publisher | Google Scholor - Glass GA, Gershon D. (1981). Enzymatic changes in rat erythrocytes withincreasing cell and donor age: loss superoxide dismutase activity associated with increases in catalytically defective forms. BiochemBiophys Res Commun, 103(4):1245-1253.
Publisher | Google Scholor - Salo DC, Pacifini RE, Davies KJN. (1998). Superoxide dismutase is preferentially degraded by a proteolytic stem from red blood cells following oxidative modification by hydrogen peroxide. Free Rad Biol Med. 1988;5(5-6):335-359.
Publisher | Google Scholor - Alejendro DB, Martha SB, Nestor OB. (1997). Superoxide dismutase, catalaseand glutathione peroxidase activities in human blood: influence of sex,age, and cigarette smoking. Clin Biochem, 30(6):449-454.
Publisher | Google Scholor - Papet I, Dardevet D, Sornet C, Be´chereau F, Prugnaud J, Pouyet C, et al. (2003). Acute phase protein levels and thymus, spleen and plasma protein synthesis rates differ in adult and old rats. J Nutr., 133(1):215-219.
Publisher | Google Scholor - Friel JK, Widness JA, Jiang T, Belkhode SL, Rebouche CJ, et al. (2002). Antioxidant status and oxidant stress may be associated with vitamin Eintakes in very low birth weight infants during the first month of life. NutrRes., 22(1-2):55-64.
Publisher | Google Scholor - Chitra S, Vidya N. (2006). Dose Dependent Effect of Piper betle Linn. LeafExtract on Erythrocytes of Experimental Mice. Sri Ramachandra, J Med., 1(1).
Publisher | Google Scholor - Al-Adhroey AH, Nor ZM, Al-Mekhlafi HM, Amran AA, Mahmud R. (2011). Antimalarial Activity of Methanolic Leaf Extract of Piper betle L. Molecules. 16(1):107-118.
Publisher | Google Scholor - Noor Aini AH, Illyana I, Musalmah M, Wan Zurinah WN. (2002). Malondialdehyde level and antioxidant enzymes activities during agingin rats with and without vitamin E supplementation. Malaysian J BiochemMol Biol. 7:50-54.
Publisher | Google Scholor - Tian L, Cai Q, Wei H. (1998). Alterations of antioxidant enzymes and oxidativedamage to macromolecules in different organs of rats during aging. FreeRadical Biol Med., 24(9):1477-1484.
Publisher | Google Scholor - Vanhoutte PM. (2005). Endothelium-derived free radicals: for worse andfor better. J Clin Invest, 107:23-25.
Publisher | Google Scholor - Powers SK, Lennon SL. (1999). Analysis of cellular responses to freeradicals: focus on exercise and skeletal muscle. Proc Nutr Soc., 58:1025-1033.
Publisher | Google Scholor - Ornoy A. (2007). Embryonic oxidative stress as a mechanism of teratogenesis with special emphasis on diabeticembryopathy. Reprod Toxicol, 24:31-41.
Publisher | Google Scholor - Del Rio D, Serafini M, Pellegrini N. (2002). Selected methodologies toassess oxidative ⁄ antioxidant status in vivo: a critical review. NutrMetab Cardiovasc Dis, 12:343-351.
Publisher | Google Scholor - Del Rio D, Stewart AJ, Pellegrini N. (2005). A review of recent studieson malondialdehyde as toxic molecule and biological markerof oxidative stress. Nutr Metab Cardiovasc Dis, 15:316-328.
Publisher | Google Scholor - Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, et al., (2007). Free radicals and antioxidants in normal physiologicalfunctions and human disease. Int J Biochem Cell Biol., 39:44-84.
Publisher | Google Scholor - Djordjevic VB. (2004). Free radicals in cell biology. Int Rev Cytol., 237:57-89.
Publisher | Google Scholor - Fridovich I. (1997). Superoxide anion radical (O2-.), superoxide dismutases, and related matters. J Biol Chem, 272:18515-18517.
Publisher | Google Scholor - Battino M, Ferreiro MS, Gallardo I, Newman HN, Bullon P. (2002). The antioxidant capacity of saliva. J Clin Periodontol., 29:189-194.
Publisher | Google Scholor - Sculley DV, Langley-Evans SC. (2002). Salivary antioxidants and periodontal disease status. Proc Nutr Soc., 61:137-143.
Publisher | Google Scholor - Canakci V, Yildirim A, Canakci CF, Eltas A, Cicek Y, Canakci H. (2007). Total antioxidant capacity and antioxidant enzymes in serum,saliva, and gingival crevicular fluid of preeclamptic women withand without periodontal disease. J Periodontol., 78:1602-1611.
Publisher | Google Scholor - Baltaciog˘lu E, Akalin FA, Alver A, Balaban F, Unsal M, Karabulut E. (2006). Total antioxidant capacity and superoxide dismutaseactivity levels in serum and gingival crevicular fluid in postmenopausal women with chronic periodontitis. J Clin Periodontol., 33:385-392.
Publisher | Google Scholor - Akalin FA, Toklu E, Renda N. (2005). Analysis of superoxide dismutaseactivity levels in gingiva and gingival crevicular fluid in patientswith chronic periodontitis and periodontally healthy controls. J Clin Periodontol., 32:238-243.
Publisher | Google Scholor - Brock GR, Butterworth CJ, Matthews JB, Chapple IL. (2004). Local andsystemic total antioxidant capacity in periodontitis and health. J Clin Periodontol., 31:515-521.
Publisher | Google Scholor - Chapple IL, Brock GR, Milward MR, Ling N, Matthews JB. (2007). Compromised GCF total antioxidant capacity in periodontitis:cause or effect? J Clin Periodontol., 34:103-110.
Publisher | Google Scholor - Tsai CC, Chen HS, Chen SL, et al. (2005). Lipid peroxidation: a possiblerole in the induction and progression of chronic periodontitis. J Periodontal Res., 40:378-384.
Publisher | Google Scholor - Marton IJ, Balla G, Hegedus C, et al. (1993). The role of reactive oxygen intermediates in the pathogenesis of chronic apical periodontitis. Oral Microbiol Immunol., 8:254-257.
Publisher | Google Scholor - Tarpey MM, Wink DA, Grisham MB. (2004). Methods for detection ofreactive metabolites of oxygen and nitrogen: in vitro and in vivoconsiderations. Am J Physiol Regul Integr Comp Physiol., 286:R431–R444.
Publisher | Google Scholor