Review Article
Scientific Update on The Treatment of Rheumatoid Arthritis: A Systematic Review and Meta-Analysis
- Bianca Gabriella de Oliveira 1*
- Melissa Alves Aires Marques 2
- Renato Barbalho Reid 3
- Nayara Maria Timóteo Gonçalves Faria 4
- Daniel Poltronieri Rangel 5
- Mariana de Souza Massetti 6
1Medical student at Universidade Salvador, Salvador, BA, Brazil.
2Medical Student at The University Iguaçu-Unig, Itaperuna, RJ, Brazil.
3Resident Hospital das Clínicas de Teresópolis Constantino Ottaviano HCTCO, General hospital, Brazil.
4Centro Universitario Serra dos Orgaos, Brazil.
5Student of the Master's Degree in Pharmaceutical Sciences at the University of Vila Velha, Brazil.
6Students at ULBRA Medicine course and members at Trauma and Emergency League- ULBRA, Brazil.
*Corresponding Author: Bianca Gabriella de Oliveira, Medical student at Universidade Salvador, Salvador, BA, Brazil.
Citation: de Oliveira B.G., Marques M.A.A., Reid R.B., Faria N.M.T.G., Rangel D.P., et al. (2024). Scientific Update on The Treatment of Rheumatoid Arthritis: A Systematic Review and Meta-Analysis, Journal of Clinical Research and Clinical Trials, BioRes Scientia Publishers. 3(4):1-11. DOI: 10.59657/2837-7184.brs.24.036
Copyright: © 2024 Bianca Gabriella de Oliveira, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: September 06, 2024 | Accepted: October 30, 2024 | Published: November 06, 2024
Abstract
Introduction: To update the treatment and management of rheumatoid arthritis. The secondary objective is to provide the orthopedic medical community with greater confidence in evidence-based management.
Methodology: Systematic review with meta-analysis carried out under the Prospective Register of Systematic Reviews (PROSPERO) database ID CRD42024570667. The search was done in MEDLINE-linked databases using the terms treatment and rheumatoid arthritis.
Results: 1,157 patients were evaluated, of whom 715 underwent oral methotrexate treatment and 442 subcutaneous. Adverse events resulting from the administration of methotrexate, whether oral or subcutaneous, do occur, in some patients they are more serious and some even give up on this therapy. Among the gastric manifestations, diarrhea was one of the most frequent, as were headaches in the neurological manifestations and local symptoms due to administration, common to the subcutaneous route.
Conclusion: If the oral route is not bearable due to gastrointestinal effects, studies suggest an appropriate switch to the subcutaneous form, to allow continuous use and maintain control of the disease.
Keywords: rheumatoid arthritis; treatment; rheumatic disease control
Introduction
Potentially a physiological modifier due to the chronic inflammatory power that can affect several joints, rheumatoid arthritis (RA) is a disease with an as yet unknown etiology, although it is autoimmune, and affects women twice as often as men. It usually starts between the ages of 30 and 40, its incidence increases with age and it develops in around 1% of the population, regardless of race [1,2].
The symptoms are extensive and begin with mild signs and occasional crises, with a long period of remission. However, it can start abruptly. The characteristics of the clinical manifestations are classic: symmetrical inflammation and joint stiffness, especially after waking up or after prolonged inactivity. Due to its inflammatory and degenerative nature, deformities occur rapidly due to the limited range of movement. The fingers tend to move slightly from their normal position towards the little finger on each hand, so that the tendons of the fingers slide out of place or other deformities develop, such as: swan neck deformity and/or button finger deformity [1-3].
Complications include: formation of synovial cysts, rheumatoid nodules, vasculitis with secondary ulcer formation due to reduced blood supply to the tissues, pleuritis, pericarditis, dyspnea, swelling of the lymph nodes (lymphadenopathy), Felty's syndrome, Sjögren's syndrome and changes in the neck, making the bones unstable and spinal cord compression. In addition to an increased risk of developing early coronary artery disease and bone disease, such as osteopenia and osteoporosis [2-4].
Diagnosis is based on the characteristic pattern of symptoms and medical professionals follow criteria established in the evaluation of the physical examination, these being involvement of the joints that are most typical of rheumatoid arthritis lasting at least six weeks. In addition, laboratory tests are requested to analyze elevated blood levels of rheumatoid factor, anti-cyclic citrullinated peptide antibodies (anti-CCP) or both, elevated C-reactive protein, elevated erythrocyte sedimentation rate (ESR) or both. Rheumatoid factor is present in 70% of people with rheumatoid arthritis and anti-CCP antibodies are positive in a further 75% of patients. Thus, the presence of anti-CCP and rheumatoid factor, especially in smokers, is a predictor of a poor prognosis [2-4,6,7].
Complementary imaging tests (radiography and magnetic resonance imaging) and joint puncture in order to analyze the synovial fluid with the characteristics of rheumatoid arthritis and to exclude other diseases that cause symptoms similar to rheumatoid arthritis. Once the diagnosis has been confirmed, it is necessary to assess the best therapeutic option for the patient [2-7]. There is a wide range of treatment options for patients, including conservative and surgical measures, in addition to the medical approach of orthopedics and rheumatology, with various therapeutic classes and interventions, so the aim of this study is to update the treatment and management of rheumatoid arthritis. Its secondary objective is to provide the orthopedic medical community with greater security in evidence-based management.
Methodology
The study is characterized as a systematic literature review, structured according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), and a PRISMA checklist was subsequently structured to analyze the results. The study protocol was drawn up and registered in the Prospective Register of Systematic Reviews (PROSPERO) database under the ID CRD42024570667. A four-phase diagram was also used to select the articles, prioritizing clarity and transparency in carrying out the systematic review and selecting the studies.
The descriptors in health sciences (DECS)/MESH TERMS were used in combination, according to the following structures: treatment and rheumatoid arthritis with “AND” and “OR” combinations. The data search took place on June 5, 2022, in the databases linked to the Medical Literature Analysis and Retrieval System Online (MEDLINE), using the Setting, Perspective, Intervention, Comparison, and Evaluation (SPICE) strategy to identify the relevant studies:
- Setting: patients with rheumatoid arthritis.
- Perspective: individuals with rheumatoid arthritis.
- Intervention: conservative treatment of rheumatoid arthritis or surgery.
- Comparison: occurrence of rheumatoid arthritis patients, diagnosis, screening and prevention.
- Evaluation: Complications following metrotexate administration.
Inclusion and Exclusion Criteria
Studies that met the following criteria were included: (1) studies with humans, age group > 18 years (2) patients diagnosed with rheumatoid arthritis (3) studies addressing rheumatoid arthritis treatment (4) studies published between 2017-2024 (5) original studies. Studies with the following criteria were excluded: (1) experimental studies using animal models (2) non-original studies - literature review (2) non-original studies - literature review (3) opinion studies (4) studies that only addressed the diagnosis of the pathology (5) studies published more than 5 years ago (6) studies that did not meet the other inclusion criteria mentioned above.
Results
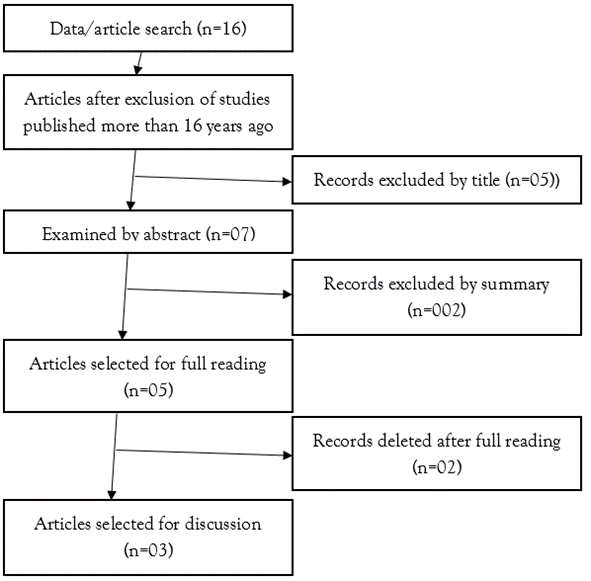
A total of 16 articles were selected during the search process, and after excluding those published more than 16 years ago, 12 remained. Analysis of the title and abstract allowed the exclusion of 07 papers that did not correspond to the objective of this study. Five articles were read in full, of which two were excluded because they did not meet the inclusion criteria, and finally three were selected for this article (Figure 1).
Figure 1: Studies selected according to PRISMA methodology.
Source: Own authorship (2024).
The four articles selected featured patients with rheumatoid arthritis who were treated with metrotexate, orally and subcutaneously. Clinical evaluation was carried out using the Disease Activity Score in 28 joints (DAS-28), the Health Assessment Questionnaire (HAQ) and adverse drug events. A total of 1,157 patients were included, of whom 715 were treated with oral metrotexate and 442 subcutaneously.
Table 1 shows the articles selected and their results (Table 1).
Table 2 shows the evaluation of adverse events [8-10].
Figure 2 shows the forest graph with the analysis of adverse events [8,10].
Table 1: Results obtained by the selected studies.
| Study | Approach | Patients F/M | Results |
| Braun et al | Administration of methotrexate via subcutaneous and oral routes | 153/222 | Study patient characteristics; Clinical efficacy; DAS28; HAD; ACR20, ACR50, ACR70; Safety of SC versus oral administration |
| Hazlewood et al | Administration of methotrexate via subcutaneous and oral routes | 148/518 | Patient population and baseline characteristics; Comparative effectiveness; DAS-28; HAQ-DI; Propensity score-adjusted models; Treatment center variability |
| Pichlmeier et al | Subcutaneous administration of methotrexate compared with oral administration | 14/51 | Symptomatology; MacNab criteria; Average operative time; Blood loss; Intraoperative complications; Length of hospital stay. |
Table 2: Results of moderate adverse events after oral and subcutaneous administration.
| Study | Sample | Middle Ages | Oral route | Subcutaneous route |
| Braun et al | 375 patients | 58 years | 79(41) | 77(41) |
| Pichlmeier et al | 116 patients | 41.9 years | 28.8 | 50 |
In the multicenter, randomized study by Braun et al [8], 187 patients in the oral administration group and 188 SC. Approximately 75% of the patients were women. In 62% the rheumatoid factor was positive. These patients had a short interval between diagnosis and randomization for the study, so the majority of patients had not yet received any rheumatic disease-modifying drugs (DMARDs) before the study, having only used anti-inflammatory drugs (NSAIDs) and/or steroids. The median DAS28 (Disease Activity Score in 28 joints) was 6.3 in the oral group and 6.1 in the SC group, indicating high levels of disease activity in both groups. At 24 weeks, the ACR20 response (criteria for 20% improvement) in the SB group was 78% and in the oral group 70% (p lessthan 0.05); the ACR70 response (criteria for 70% improvement) was also higher in the SB group than in the oral group (41% versus 33%; p lessthan 0.05). No significant difference was found between the proportion of patients who achieved an ACR50 response (criteria 50% improvement; 62% SB versus 59% oral group). As for the joints, the number of swollen joints was lower in the SC group compared to the oral group (p=0.04), as was the number of tender joints (p=0.08). The mean HAQ (Health Assessment Questionnaire) score was slightly lower in the CS group than in the oral group (0.4 versus 0.5), but the difference was not significant. In addition, the median DAS-28 score was also lower in the SC group than in the oral group (3.3 versus 3.7) after 24 weeks. With regard to administration safety, 66% of patients treated via the SC route reported one adverse event (AE) during the study, compared to 62% of the other group. As for a serious event, 5.7% of the SC group and 4.3% of the oral group. The frequency of moderate or serious AEs reported was higher in the oral group (3%), diarrhea was also reported more frequently in patients treated with oral MTX (6.9% versus 2.6% for SC). Loss of appetite was higher in the SC group (7.3% versus 3.2% for oral).
In the multicenter, prospective cohort study by Hazlewood et al [9], 417 patients received oral treatment and 249 received SC treatment. Patients in the SC group had more comorbidities and were prescribed a higher dose of MTX. They were less likely to receive oral corticosteroids and more likely to receive intramuscular or intra-articular corticosteroids. They were also younger and often had erosions on their initial X-rays. The initial DAS-28 scores in the oral group were 5.5 and, in the SC, they were 5.5(1.4; p=0.86), while the HAQ-DI scores were 1.1(0.69; p=0.52) for both groups. Over the course of the study, DAS-28 scores decreased significantly more in the CS group at 03, 06 and 09 months, but not at 12 months, with a mean score of 0.38 (p=0.001) and a greater likelihood of remission over the first year. In the models adjusted for propensity score, the significant association between MTX route and treatment failure remained (HR for treatment failure: 0.55; 0.39 and 0.79). Regarding the models adjusted by propensity score, when restricting the analysis to centers in which 25% of patients were treated orally and SC, there was no significant association between the route of MTX and treatment failure.
The randomized study by Pichlmeier et al [10], 57 patients in the oral group and 59 in the SC, 11 subjects were excluded from the study, 03 for not having been treated and 08 for discontinuing after the first dose of MTX, as for the reasons for discontinuation: 02 for personal reasons; 01 suffered an adverse event that made it undesirable to continue and 05 due to test results. Regarding the pharmacological characteristics, rapid absorption of MTX was observed in the SC group compared to the oral group, Cmax (maximum plasma concentration) levels reached between 0.5 and 2.5 hours after administration for both groups; AUC0-t (time t) was higher in the SC group. The most frequently reported AEs were gastrointestinal (22 patients), followed by nervous system disorders (12 patients) and general disorders and administration site conditions (10 patients). Gastrointestinal disorders were more common in the oral route group compared to SC, occurring in 28.1% of subjects versus 15.3% of subjects, respectively. The most reported event was diarrhea (12 subjects). Patients reported nervous system symptoms and headaches in 11.9% of the SC group and 10.5% of the oral group. Due to the number of reactions at the application site, general disorders and conditions at the administration site were higher in the SC group than in the oral group. However, no individual showed swelling or redness after the MTX injection; 02 patients developed a small hematoma at the application site, and 05 patients in the SC group reported slight pain or a burning sensation. As for the results of the laboratory tests, they revealed a reversible increase in transaminases in several individuals after administration via SC and/or oral, 02 patients in the oral group recorded a significant increase in transaminases.
Discussion
Adverse events resulting from the administration of metrotexate, whether oral or subcutaneous, occur, in some patients they are more serious and some even give up on this therapy. Diarrhea among the gastric manifestations was one of the most frequent, headaches in the neurological ones and local symptoms due to administration, common to the subcutaneous route [8,10].
The increase in systemic inflammation that occurs with physiological ageing leads to a change in body composition due to an increase in fat mass and sarcopenia, resulting in impaired balance and falls, which are associated with deleterious outcomes. Physical activity has anti-inflammatory effects through lipolysis, increasing the anti-inflammatory and regulatory properties of the immune system and increasing the interleukin produced in muscle [11].
Methotrexate (MTX) is an antifolate drug with anti-inflammatory, immunomodulatory and antiproliferative effects, by inhibiting several key enzymes involved in folate, methionine and adenosine. This drug has been established as an effective, safe and inexpensive first-line treatment for immune-mediated inflammatory diseases. Thus, because of its relatively low cost and favorable efficacy profile, MTX is considered the first-choice treatment for rheumatoid arthritis (RA). However, the choice of route of administration is still under discussion by experts, as depending on the route MTX can significantly affect its bioavailability, thus influencing both its efficacy and tolerability [11-13].
Oral methotrexate is the most frequently prescribed treatment for RA and has been used in the clinic for a long time. This is mainly due to the convenience and low costs of oral administration. Recently, some studies have shown that higher doses improve clinical results. However, as the dose increases, oral MTX shows a saturation effect and may show decreased bioavailability and frequent gastrointestinal side effects, such as nausea, vomiting, changes in liver function tests, poor taste, dyspeptic symptoms, diarrhea and stomatitis, which in turn limit the optimal use of the drug [12,14,15].
Methotrexate administered subcutaneously has attracted a lot of attention from rheumatologists in recent years. This route has well-absorbed and well-tolerated clinical results, which are effective at high doses. Thus, with regard to the tolerability of subcutaneous MTX at the injection site, studies have shown that the administrations revealed mild to moderate pain, but were generally well tolerated. In addition, according to some studies, this route of administration has shown a lower occurrence of gastrointestinal side effects, and is often prescribed for those patients who do not tolerate oral methotrexate very well [12,14].
That said, studies indicate greater efficacy and better tolerability with subcutaneous MTX compared to oral MTX in the treatment of rheumatoid arthritis. Furthermore, from a pharmacokinetic point of view, subcutaneous MTX has a higher bioavailability compared to the oral route. From a therapeutic point of view, subcutaneous MTX is superior to oral MTX in terms of clinical efficacy and subcutaneous MTX has better tolerability in terms of gastrointestinal side effects. Because of this, subcutaneous administration has begun to be taken into consideration for the start of treatment and should be tried in patients with a lack of response or intolerance to oral MTX [13,16].
Conclusion
It is concluded that if the oral route is not bearable due to gastrointestinal effects, studies suggest an appropriate switch to the subcutaneous form, in order to allow continuous use and maintain control of the disease. Overall, subcutaneous MTX is characterized by greater bioavailability, greater clinical efficacy and a better tolerability profile, making it a key treatment for optimizing the treatment of rheumatoid arthritis.
References
- Cevenini E, Monti D, Franceschi C. (2013). Inflamm-Ageing. Curr Opin Clin Nutr Metab Care. 16(1):14-20.
Publisher | Google Scholor - Stevens JA, Corso PS, Finkelstein EA, Miller TR. (2006). The Costs of Fatal and Non-Fatal Falls Among Older Adults. Inj Prev. 12(5):290-295.
Publisher | Google Scholor - Hurkmans E, Van Der Giesen FJ, Vliet Vlieland TP, Schoones J, Van Den Ende EC. (2009). Dynamic Exercise Programs (Aerobic Capacity and/or Muscle Strength Training) in Patients with Rheumatoid Arthritis. Cochrane Database Syst Rev. 4:CD006853.
Publisher | Google Scholor - Rydwik E, Frändin K, Akner G. (2004). Effects of Physical Training on Physical Performance in Institutionalised Elderly Patients (70+) With Multiple Diagnoses. Age Ageing. 33(1):13-23.
Publisher | Google Scholor - Buitinga L, Braakman-Jansen LM, Taal E, Van De Laar MA. (2012). Future Expectations and Worst-Case Future Scenarios of Patients with Rheumatoid Arthritis: A Focus Group Study. Musculoskeletal Care. 10(4):240-247.
Publisher | Google Scholor - Lange E, Kucharski D, Svedlund S, et al. (2019). Effects of Aerobic and Resistance Exercise in Older Adults with Rheumatoid Arthritis: A Randomized Controlled Trial. Arthritis Care Res. 71(1):61-70.
Publisher | Google Scholor - Ellegaard K, Von Bülow C, Røpke A, et al. (2019). Hand Exercise for Women with Rheumatoid Arthritis and Decreased Hand Function: An Exploratory Randomized Controlled Trial. Arthritis Res Ther. 21(1):158.
Publisher | Google Scholor - Braun J, Kästner P, Flaxenberg P, Währisch J, Hanke P, et al. (2008). Comparison of the Clinical Efficacy and Safety of Subcutaneous Versus Oral Administration of Methotrexate in Patients with Active Rheumatoid Arthritis: Results of A Six-Month, Multicenter, Randomized, Double-Blind, Controlled, Phase IV Trial. Arthritis Rheum. 58(1):73-81.
Publisher | Google Scholor - Hazlewood GS, Thorne JC, Pope JE, Lin D, Tin D, et al. (2016). The Comparative Effectiveness of Oral Versus Subcutaneous Methotrexate for The Treatment of Early Rheumatoid Arthritis. Ann Rheum Dis. 75(6):1003-1008.
Publisher | Google Scholor - Pichlmeier U, Heuer KU. (2014). Subcutaneous Administration of Methotrexate with A Prefilled Autoinjector Pen Results in A Higher Relative Bioavailability Compared with Oral Administration of Methotrexate. Clin Exp Rheumatol. 32(4):563-571.
Publisher | Google Scholor - Torres RP, Santos FP, Branco JC. (2022). Methotrexate: Implications of Pharmacogenetics in The Treatment of Patients with Rheumatoid Arthritis. ARP Rheumatol. 1(3):225-229.
Publisher | Google Scholor - Vermeer E, Hebing RCF, Van De Meeberg MM, Lin M, De Meij TGJ, et al. (2023). Oral Versus Subcutaneous Methotrexate in Immune-Mediated Inflammatory Disorders: An Update of The Current Literature. Curr Rheumatol Rep. 25(12):276-284.
Publisher | Google Scholor - Bianchi G, Caporali R, Todoerti M, Mattana P. (2016). Methotrexate and Rheumatoid Arthritis: Current Evidence Regarding Subcutaneous Versus Oral Routes of Administration. Adv Ther. 33(3):369-378.
Publisher | Google Scholor - Li D, Yang Z, Kang P, Xie X. (2016). Subcutaneous Administration of Methotrexate at High Doses Makes a Better Performance in The Treatment of Rheumatoid Arthritis Compared with Oral Administration of Methotrexate: A Systematic Review and Meta-Analysis. Semin Arthritis Rheum. 45(6):656-662.
Publisher | Google Scholor - Borman P, Demir G, Kaygısız F, Okumuş M. (2014). Subcutaneous (SC) Methotrexate (MTX) Is Better and Well-Tolerable Than Oral MTX In Rheumatoid Arthritis Patients, Switched from Oral to SC Administration Due to Gastrointestinal Side Effects. Open Rheumatol J. 8:18-19.
Publisher | Google Scholor - Mouterde G, Baillet A, Gaujoux-Viala C, Cantagrel A, Wendling D, et al. (2011). Optimizing Methotrexate Therapy in Rheumatoid Arthritis: A Systematic Literature Review. Joint Bone Spine. 78(6):587-592.
Publisher | Google Scholor