Research Article
Relationship and Prevalence of Uterine Scar Defects (Niches) With Cases of Abnormal Uterine Bleeding
- A. Abd El-Samie 1
- Rana A. Fawzy 2
- Sahar MY. El-Baradie 1
- Laila E. Abd El-Fattah 1
- Mohamed K. Etman 1*
1Obstetrics and gynecology department, Faculty of Medicine, Fayoum University, Egypt.
2Obstetrics and gynecology department, El-Wasta Central Hospital, Egypt.
*Corresponding Author: Mohamed K. Etman, Obstetrics and gynecology department, Faculty of Medicine, Fayoum University, Egypt.
Citation: A.A. El-Samie, Rana A. Fawzy, S.M.Y. El-Baradie, L.E.A. El-Fattah, Mohamed K. Etman. (2024). Relationship And Prevalence of Uterine Scar Defects (Niches) With Cases of Abnormal Uterine Bleeding, Clinical Obstetrics and Gynecology Research, BRS Publishers. 3(1):1-8. DOI: 10.59657/2992-9725.brs.24.011
Copyright: © 2024 Mohamed K. Etman, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: February 05, 2024 | Accepted: March 26, 2024 | Published: April 02, 2024
Abstract
Background: Cesarean scar defects are increasing nowadays with a great impact on women’s health. This study was conducted to evaluate the relationship and prevalence of uterine scar defects in women with abnormal uterine bleeding.
Materials and Methods: This prospective cohort study recruited 150 patients with menstrual irregularities after at least one cesarean delivery who were selected randomly from the outpatient Obstetric Clinic at Fayoum university hospital from (March /2020 to March / 2021). All included women were assessed using transvaginal ultrasound and transabdominal ultrasound for measurement of the size, number, degree, shape, depth, and myometrial thickness of the uterine scar defect and residual myometrium.
Results: The prevalence of Niche was 34.7%. Parity and the number of previous cesarean deliveries were significantly higher in women with diagnosed uterine scar defects; (p-value= 0.001 each). The number of previous cesarean deliveries showed a significant positive linear correlation with the width (r= 0.412, p=0.002) and depth (r=0.359, p=0.009) of the uterine scar defect. Residual myometrium was significantly smaller in women with menorrhagia, metrorrhagia, and postmenstrual bleeding (p=0.039) (p=0.031).
Conclusion: Cesarean sections contribute to the presence of Niche and are related to it in terms of depth and width. The existence of a uterine niche is linked to postmenstrual and postcoital bleeding.
Keywords: cesarean sections; uterine scar defect; niche; transvaginal ultrasound
Introduction
Cesarean section (CS) is by far the most common major operation in the world, and its incidence is rising, and any long-term complications are becoming more important [1]. Long-standing gynecological complications following CS, such as chronic pelvic pain, dyspareunia, dysmenorrhea, postmenstrual spotting, and even infertility, have become increasingly known over the past two decades [2]. Furthermore, long-term obstetric consequences seem to be on the rise in the form of a variety of disorders, including cesarean scarring, ectopic pregnancy, an elevated rate of placenta previa, varying grades of adherent anterior placenta previa, and placenta accreta, all of which are related to significant maternal morbidity and mortality [3]. Several professional obstetricians believe there is a disproportionate increase in the niche prevalence; increased diagnosis and cesarean rate do not fully justify this increase in cesarean uterine scars (Niche). The literature on the symptoms of CS defect and treatment options has exploded in the last decade [4]. Several studies on cesarean scar defect have recently been published, Thurmond et al., who diagnosed Niche with sonohysterography, named it isthmocele [5]. The most frequently used procedures to diagnose previous cesarean delivery scar defect (PCDS) are hysteroscopy and transvaginal ultrasound (TVS). PCDS is attributed to poor healing of the myometrium at the site of the uterine incision [4]. On TVS, scar defect can be described as a triangular anechoic space at the uterine anterior lower segment [6] or through direct visualization under hysteroscopy at the lower segment [7].
The gynecological complications of CS, including abnormal uterine bleeding (AUB), chronic pelvic pain, dysmenorrhea, and secondary infertility, recently got much attention [8]. Abnormal uterine bleeding (AUB) changes followed by CS have different forms as postmenstrual, intermenstrual, postcoital, and heavy menstrual bleeding [9], and the size of isthmocele has been correlated to the postmenstrual spotting [4]. Previous research suggests a relationship between defects and irregular uterine bleeding after a cesarean section [10]. While performing TVS, Fabres et al. aspirated a brownish-colored fluid that had collected in a scar defect [11]; Raimondo et al. also showed blood clots in a scar defect that were gradually released under hysteroscopy [12], suggesting that accumulation of blood is a possible mechanism of abnormal uterine bleeding associated with PCDS. While previous studies have described PCDS using TVS, evidence is scarce on how these recognizable CS defects are linked to irregular uterine bleeding symptoms [13]. Accordingly, we prospectively investigate uterine scar defects prevalence and relationship to abnormal uterine bleeding in the current study.
Materials and Methods
This was a prospective cohort study on 150 women with menstrual abnormalities after a minimum of one CS who were chosen randomly from the outpatient Obstetric Clinic at Fayoum university hospital from March /2020 to March /2021. Women were recruited according to the following inclusion and exclusion criteria. Inclusion criteria: a) Age: 20-45 years, b) patients with at least one CS delivery, c) Patients with any menstrual irregularities after CS. Menstrual irregularities were defined as a) Heavy menstrual bleeding/menorrhagia; defined as excessive menstrual blood loss interfering with women's physical, social, emotional, and quality of life [14], b) Bleeding/ spotting episode/metrorrhagia; defined as one or more consecutive days during which blood loss has been reported [15], and c) Polymenorrhea; defined as intervals of up to 21 days [16], d) postcoital bleeding, and e) postmenstrual spotting; defined as brownish discharge lasting for ≥ 2 days after cessation of menstruation [19]. Exclusion criteria: a) women using the copper intrauterine contraceptive device, b) women using LNG- IUS, c) women using hormonal contraceptive methods, d) women with coagulation disorders, e) any pelvic organic lesions (uterine fibroids, adenomyosis, ovarian cysts or tumors, and cervical polyps), f) any other causes of abnormal uterine bleeding (PID, inflammation or ulceration secondary to foreign bodies, vaginal lesions, infections, laceration, or trauma). All enrolled women were evaluated as complete history taking, particularly for (menstrual history, obstetric history, medical illness, and vaginal bleeding). Transvaginal ultrasound (TVS) using (Philips Medical Systems) ultrasound apparatus with the 2D endovaginal probe with frequency 9.3 MHz and trans abdominal probe with frequency 7 MHz for assessment of the size, number, degree, shape, depth, and myometrial thickness of the uterine scar defect and residual myometrium. Women were examined at day 3-5 of the cycle. All examinations were done by the same researcher to avoid interobserver variability.
Statistical analysis
The collected data were coded, entered, and analyzed using the IBM software statistical package for social sciences (SPSS) (version 25). For categorical variables, descriptive statistics were in the form of frequency and percentage, while for numerical variables, in the form of mean and standard deviation. (mean ± SD). The proper statistical significance measures were used: (Independent Sample t-test, Chi-Square (χ2) test, and Pearson's correlation analysis; {r- values: 0 to 0.3 positive or negative (slight), 0.3 to 0.7 (moderate) and 0.7 to 1 (strong). Statistical significance was described at a p-value of less than or equal to 0.05.
Results
The prevalence of Niche was (34.7%) in our studied women. Age was insignificantly higher among women with Niche than women without; (p-value>0.05). Higher parity was associated with a higher prevalence of Niche. Parity ranged from (1) to (6) with an average of (3.27 ±1.19) in women with diagnosed uterine scar defect (Niche) vs. an average parity of (2.34 ±1.30) times in women without Niche; (p-value= 0.001). The prevalence of Niche was significantly increased with an increased number of previous CS; (p-value= 0.001). Duration since the last CS showed a non-statistically significant difference between both groups; (p-value>0.05). The most common bleeding pattern was menorrhagia in 62% of the studied population. Metrorrhagia was significantly more prevalent in women with uterine scar defect (23.1% vs. 9.2%) than those without; (p-value=0.020). Postcoital bleeding was significantly more prevalent in women with uterine scar defect (19.2% vs. 6.1%) than those without; (p-value=0.016). Postmenstrual bleeding was significantly more prevalent in women with uterine scar defect (32.7% vs. 12.2%) in women with and without Niche, respectively (p-value=0.003) (Table 1).
Table 1: Baseline data among studied participants according to the prevalence of uterine scar defect Niche; N=150.
| Prevalence of Niche | p-value | ||||
| No Niche N= 98 | Niche N= 52 | Total N= 150 | |||
| Maternal Age; (years) | |||||
| Mean ±SD | 30.28 ±5.05 | 32.19 ±7.23 | 30.94 ±5.94 | 0.061 | |
| 95% CI for Mean (Lower Bound – Upper Bound) | 29.27 – 31.30 | 30.18 – 34.20 | 29.99 – 31.91 | ||
| Range (Mini – Max) | 20 – 44 | 23 – 45 | 20 – 45 | ||
| Parity; | |||||
| Mean ±SD | 2.34 ±1.30 | 3.27 ±1.19 | 2.66 ±1.34 | 0.001* | |
| 95% CI for Mean (Lower Bound – Upper Bound) | 2.08 - 2.60 | 2.94 - 3.60 | 2.44 - 2.88 | ||
| Range (Mini – Max) | 1 - 6 | 1 - 6 | 1 - 6 | ||
| Number of Previous CS; | |||||
| Mean ±SD | 2.06 ±1.03 | 3.19 ±1.17 | 2.45 ±1.21 | 0.001* | |
| 95% CI for Mean (Lower Bound – Upper Bound) | 1.85 - 2.27 | 2.87 - 3.52 | 2.26 - 2.65 | ||
| Range (Mini – Max) | 1 – 5 | 1 – 5 | 1 – 5 | ||
| Duration Since Last CS; (years) | |||||
| Mean ±SD | 2.85 ±1.80 | 3.46 ±2.53 | 3.06 ±2.09 | 0.089 | |
| 95% CI for Mean (Lower Bound – Upper Bound) | 2.49 - 3.21 | 2.76 - 4.17 | 2.73 - 3.40 | ||
| Range (Mini – Max) | 0.5 – 9 | 0.5 – 10 | 0.5 – 10 | ||
*p-value ≤0.05 is considered statistically significant.
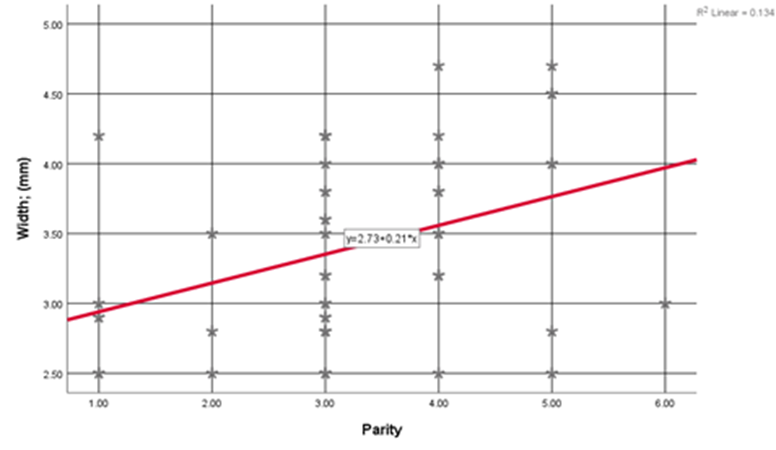
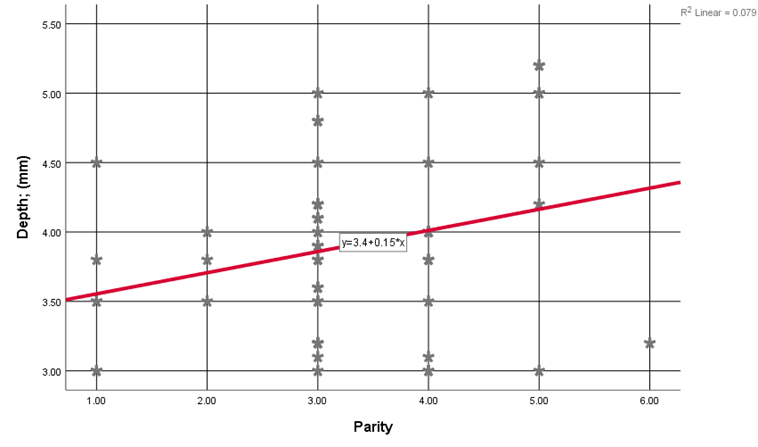
Triangular Niche was the most prevalent shape in 26.9% of cases, followed by Semi-Circular (23.1%), Linear (23.1%), Oval (23.1%), and finally, Wedge-shaped Niche (3.8%) of cases. Niche width ranged from (2.50) to (4.70) with an average of (3.40 ±0.67) mm, depth ranged from (3.00) to (5.20) with an average of (3.90 ±0.64) mm, and the residual myometrium ranged from (4.10) to (7.80) with an average of (6.30 ±1.03) mm. Women's Age showed a non-statistically significant linear correlation with uterine scar defect (Niche) measurements [width (r= 0.152, p= 0.281), depth (r= 0.212, p=0.132), and residual myometrium (r= -0.251, p= 0.072)]. Parity showed a positive significant linear correlation with width (r= 0.365, p=0.008) (Figure 1) and depth (r=0.282, p=0.043) of the uterine scar defect (Figure 2) among studied women. Previous CS showed a positive slight to moderate significant linear correlation with width (r= 0.412, p=0.002) and depth (r=0.359, p=0.009) of the uterine scar defect among studied women. Duration Since Last CS showed a non-statistically significant linear correlation with uterine scar defect (Niche) measurements (width, depth, and residual myometrium); (p-values>0.05).
Figure 1: Correlation between uterine scar defect (Niche) Width and Parity among studied women.
Figure 2: Correlation between uterine scar defect (Niche) depth and parity among studied women.
Table 2 demonstrates the relation of uterine bleeding pattern with niche measurements in the Niche group. Residual myometrium (mm) was significantly smaller in women with menorrhagia as compared with women without (6.56 ±1.04 vs. 5.96 ±0.98), (p=0.039). Niche width and depth showed a non-statistically significant difference with menorrhagia uterine bleeding. Residual myometrium (mm) was significantly smaller in women with metrorrhagia as compared with women without (6.87 ±1.03 vs. 6.14 ±0.89), (p=0.031). Niche depth was significantly smaller in women with metrorrhagia than those without (3.58 ±0.63 vs. 4.00 ±0.58), (p=0.045), but niche width showed a non-statistically significant difference with menorrhagia uterine bleeding. None of the niche measurements (width, depth, or residual myometrium) showed a statistically significant association with Post-Coital Bleeding (p-values>0.05). Residual myometrium (mm) was significantly smaller in women with postmenstrual bleeding than in women without. Niche width and depth were significantly larger with postmenstrual bleeding uterine bleeding symptoms; (p-values lessthan 0.01).
Table 2: Relation of Uterine bleeding pattern with niche measurements in the Niche group; (N= 52):
| Mean | SD | p-value | |||
| Menorrhagia | Width; (mm) | No | 3.48 | 0.69 | 0.528 |
| Yes | 3.36 | 0.66 | |||
| Depth; (mm) | No | 4.08 | 0.66 | 0.081 | |
| Yes | 3.77 | 0.60 | |||
| Residual Myometrium, (mm) | No | 6.56 | 1.04 | 0.039* | |
| Yes | 5.96 | 0.98 | |||
| Metrorrhagia | Width; (mm) | No | 3.45 | 0.66 | 0.441 |
| Yes | 3.28 | 0.74 | |||
| Depth; (mm) | No | 3.58 | 0.63 | 0.045* | |
| Yes | 4.00 | 0.58 | |||
| Residual Myometrium, (mm) | No | 6.87 | 1.03 | 0.031* | |
| Yes | 6.14 | 0.89 | |||
| Post-Coital Bleeding | Width; (mm) | No | 3.41 | 0.68 | 0.968 |
| Yes | 3.40 | 0.67 | |||
| Depth; (mm) | No | 3.90 | 0.65 | 0.914 | |
| Yes | 3.88 | 0.66 | |||
| Residual Myometrium, (mm) | No | 6.32 | 1.00 | 0.805 | |
| Yes | 6.23 | 1.24 | |||
| Post-Menstrual bleeding | Width; (mm) | No | 3.14 | 0.55 | <0> |
| Yes | 3.94 | 0.56 | |||
| Depth; (mm) | No | 3.62 | 0.54 | <0> | |
| Yes | 4.46 | 0.42 | |||
| Residual Myometrium, (mm) | No | 6.65 | 0.89 | <0> | |
| Yes | 5.58 | 0.94 | |||
*p-value ≤0.05 is considered statistically significant.
Discussion
The prevalence of a niche as detected by TVS was 34.7% in our studied population; this prevalence is higher but comparable with that of (24.0%) (18) and (19.4%) (19) in previous studies using the same methodology. However, this prevalence was lower than the reported prevalence rate of (46.3 %) in a study where women were assessed by saline contrast sonohysterography (SHG) (4). Conversely, some authors reported a much lower prevalence of 6.9% (20). These disparities in prevalence may have been caused by a discrepancy in niche definitions, level of understanding, and methods used in diagnosis. Although several risk factors have been established, most authors agree that multiple cesarean deliveries are the leading cause of uterine scarring [21]. Our study has confirmed this observation, higher parity was associated with a higher prevalence of Niche, and we also found that the prevalence of Niche was increased with an increased number of previous CS. Duration since the last CS showed a non-statistically significant difference between women with uterine scar defect (Niche) and women without it. A connection between several previous CS and scar defects has been described in earlier studies [4, 18, 22]. A scenario where repeated trauma to a wound can interrupt the normal healing process is similar to this event [23]. Adhesion formation with the abdominal wall, on the other hand, forces the uterine scar against the abdominal wall, exerting a counteracting force in the opposite direction of uterine scar tissue, retracting and reducing wound healing [24]. All women included in the current study have menstrual abnormalities after CS. The most reported bleeding pattern was menorrhagia in 62%. Metrorrhagia, postcoital, and postmenstrual bleeding were significantly more prevalent in women with uterine scar defects. The relation between a cesarean scar defect and irregular uterine bleeding may be due to the accumulation of menstrual blood in the defect and the presence of fibrotic tissue underneath it that could obstruct proper blood drainage and in-situ blood output by newly formed blood vessels [22].
One year after CS, postmenstrual spotting was linked to the presence of Niche identified with SHG six months after CS, Postmenstrual spot was confirmed by one in every five women with Niche (20%), compared with one in every twelve women without Niche (8.3%) [4]. According to Bij de Vaate et al., postmenstrual spotting affects one-third of women with Niche, opposite to one-seventh of women without Niche [25]. However, since participants were recruited several months after the CS, it is possible that symptomatic patients were chosen. We believe that selection bias had a minor impact in the current study, which may explain the lower rate of postmenstrual spotting observed. T1-weighted images with an abundance of fatty tissue demonstrated menstrual blood retention in the uterine scar explaining postmenstrual bleeding [26]. This retention has been hypothesized to be intermittently expelled [27] and may cause chronic pelvic pain and dysmenorrhea symptoms by inducing an inflammatory environment [26, 27]. The most common defects are triangular or semi-circular, but there are also round, oval, droplet-shaped, and inclusion cysts [28]. An inward protrusion, an outward protrusion, and an inward retraction are all different forms of niches [4]. In the current study, triangular Niche was the most prevalent shape in (26.9%) of cases, followed by semi-circular, linear, oval, and finally, wedge-shaped Niche of cases. Those shapes were slightly different from the reported by Bij de Vaate et al., where the most common shapes were semi-circular, triangular, droplet-shaped, and inclusion cysts [25]. The shape of the Niche had no significant association with abnormal uterine bleeding in this study.
The number of previous CS and parity were found to have a significant positive linear correlation with niche width and depth in our sample. Several authors have indicated that the defect is significant when there have been several previous caesareans, likely due to increased adhesion formation, which could predispose to a cesarean scar defect due to scar tissue retraction. [20, 21]. In the current study, women who presented with a large niche reported postmenstrual bleeding even more often. Niche width and depth were significantly larger with postmenstrual bleeding uterine bleeding symptoms; (p-values lessthan 0.01). Residual myometrium (mm) was significantly smaller in women with postmenstrual bleeding than in women without. Postmenstrual spotting was found in 20% of women with Niche compared to 8.3% of women without Niche, with 3.34 OR for significant defects [4]; this was consistent with the findings of previous prospective studies [10, 25]. The connection between niche size and postmenstrual bleeding supports the theory that spotting is caused by blood accumulated within the niche pouch [28]. Considering symptomatic patients, the strong correlation of niche defects with menstrual bleeding disorders prompts us to suggest invasive surgical interventions to manage those symptoms. Nevertheless, the effect of hysteroscopic niche resection on postmenstrual bleeding has only been investigated in one randomized controlled clinical trial [29]. Conduct clinical trials for the treatment of symptomatic niches.
Strength and limitations:
The current study has some potential limitations, the lack of data on how CS was performed; this was because all the included women were referrals, with the CS being performed by many surgeons at various institutes. Suturing procedures, CS indications, and post-Cesarean cases were all lacking information. Consequently, how these issues influence niche development is not known. As a result of the lack of standardization in ultrasound screening timing, data on the initiation of symptoms after the most recent CS cannot be drawn from this analysis. An additional significant limitation of our analysis is a lack of a validated tool to evaluate postmenstrual bleeding. Only severe menstrual forms of AUB have established validated patient-reported outcome measures [30]. These forms are used to determine the amount of lost blood that is unsuitable for the present study.
Conclusion
Cesarean sections contribute to the presence of Niche and are related to it in terms of depth and width. The existence of a uterine niche is linked to postmenstrual and postcoital bleeding.
List of abbreviation
CS: Cesarean section
PCDS: Previous cesarean delivery scar defect
TVS: Transvaginal ultrasound
AUB: Abnormal uterine bleeding
LNG- IUS: Levonorgestrel intrauterine system
PID: Pelvic inflammatory disease
SHG: Sonohysterography
Declarations
Ethical approval and consent to participate:
This study was conducted after approval of the research ethics committee of the faculty of medicine, Fayoum University. All procedures performed in the study were following the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. Informed written consent was obtained from all participants before enrollment in the study.
Consent for publication:
Not applicable.
Availability of data and materials:
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article.
Competing interest:
None.
Funding:
Self-funded research.
Authors' contributions:
AAA: Protocol/project development, Data collection and management, manuscript writing/editing.
RAF: Data analysis, manuscript writing and editing.
SMYE: Data management, Manuscript writing/editing.
LEA: Protocol/project development, Data analysis, Manuscript writing/editing.
MKE: Data management, Manuscript writing/editing. All authors have read and approved the manuscript.
Acknowledgment:
not applicable.
References
- Deenan JCW HC, Verschuuren JJGM, Verbeek ALM. (2015). The epidemiology of neuromuscular disorders: a comprehensive overview of the literature. Journal of neuromuscular diseases. 2: 73-85.
Publisher | Google Scholor - Leveno KJ, Spong CY, Dashe JS, Casey BM, Hoffman BL, et al. (2018). Williams Obstetrics, 25th Edition. McGraw-Hill Education.
Publisher | Google Scholor - Inoue KI, Tsugawa J, Fukae J, Fukuhara K, Kawano H, et al. (2020). Myasthenia Gravis with Anti-MuscleSpecific Tyrosine Kinase Antibody during Pregnancy and Risk of Neonatal Myasthenia Gravis: A Case Report and Review of the Literature. Case Rep Neurol. 12(1):114-120.
Publisher | Google Scholor - Djelmis J, Sostarko M, Mayer D, et al. (2020). Myasthenia gravis in pregnancy: Report on 69 cases. Eur J Obstet Gynecol Reprod Biol. 104: 21-25.
Publisher | Google Scholor - Ferrero S, Pretta S, Nicoletti A, et al. (2005). Myasthenia gravis: Management issues during pregnancy. Eur J Obstet Gynecol Reprod Biol. 121(2): 129-138.
Publisher | Google Scholor - Connell F. Myasthenia Gravis. En, Atlee JL. (1999). Complications in anesthesia. Philadelphia, PA: WB Saunders; p. 490-493.
Publisher | Google Scholor - Briggs ED, Kirsch JR. (2003). Anesthetic implications of neuromuscular disease. J Anesth. 17: 177-185.
Publisher | Google Scholor - Bansal R, Goyal M, Modi M. (2018). Management of myasthenia gravis during pregnancy. Indian J Pharmacol. 50(6): 302.
Publisher | Google Scholor - Plauché WC. (1991). Myasthenia gravis in mothers and their newborns. Clin Obstet Gynecol. 34: 82-99.
Publisher | Google Scholor - Munnur U, Karnad DR, Bandi VDP et al. (2005). Critically ill obstetric patients in an American and an Indian public hospital: Comparison of case-mix, organ dysfunction, intensive care requirements and outcomes. Intensive Care Med. 31: 1.087–1.094.
Publisher | Google Scholor - Stafford IP, Dildy GA. (2005). Myasthenia gravis and pregnancy. Clin Obstet Gynecol. 48: 48-56.
Publisher | Google Scholor - Uncu G, Küçükerdogan I, Ozan H, et al. (1995). Pregnancy and myasthenia gravis. A case report. Clin Exp Obstet Gynecol; 22: 145-147.
Publisher | Google Scholor - Namba T, Brown SB, Grob D. (1970). Neonatal myasthenia: report of two cases and review of the literature. Pediatrics. 45: 488-504.
Publisher | Google Scholor - Kim JM, Mangold J. (1989). Sensitivity to both vecuronium and neostigmine in a sero- negative myasthenic patient. Br J Anaesth. 63: 497.
Publisher | Google Scholor - Nilsson E, Meretoja OA. (1990). Vecuronium dose-response and maintenance requirementsin patients with myasthenia gravis. Anesthesiology. 73: 28- 32.
Publisher | Google Scholor - Hoff JM DA, Gilhus NE. (2003). Myasthenia gravis consequences for pregancy, delivery, and the newborn. Neurology. 61: 1362-1366.
Publisher | Google Scholor - Eden RD GS. (1983). Myasthenia gravis and pregnancy: A reappraisal of thymectomy. Obstetrics and Gynecology. 62(3):328-333.
Publisher | Google Scholor - Ducci RD, Lorenzoni PJ, Kay CSK, et al. (2017). Clinical follow-up of pregnancy in myasthenia gravis patients. Neuromuscul Disord, NMD; 27: 352-357.
Publisher | Google Scholor - Perucca PE, Cuellar JE, Ricci AP, et al. (2006). Miastenia Gravis: Embarazo e Impacto Perinatal. Rev Chil Obstet Ginecol; 71: 201-206.
Publisher | Google Scholor - Blencowe H, Cousens S, Oestergaard MZ, et al. (2012). National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet; 379: 2162-2172.
Publisher | Google Scholor