Case Report
Ramipril induced Erythematous rash in an Adult Hypertensive Patient: A case report
1Student, Phase III Part 2, Christian Medical College & Hospital, Ludhiana.
2Assistant Professor, Department of Pharmacology, Christian Medical College & Hospital, Ludhiana.
3Pharmacovigilance Associate, AMC, Christian Medical College & Hospital, Ludhiana.
4Professor & Head, Department of Pharmacology, Christian Medical College & Hospital, Ludhiana.
*Corresponding Author: Girish Joseph, Assistant Professor, Department of Pharmacology, Christian Medical College & Hospital, Ludhiana.
Citation: Goyal G., Bhatti N., Kaur P., Joseph G., Badya D., et al. (2024). Ramipril induced Erythematous rash in an Adult Hypertensive Patient: A case report. Clinical Case Reports and Studies, BioRes Scientia Publishers. 6(6):1-10. DOI: 10.59657/2837-2565.brs.24.167
Copyright: © 2024 Girish Joseph, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: July 29, 2024 | Accepted: August 20, 2024 | Published: August 29, 2024
Abstract
Background: Ramipril is being used as monotherapy or combination therapy for patients with Hypertension. Many cutaneous adverse drug reactions have been reported worldwide with the suspect drug including Hereditary Angioneurotic edema, DRESS Syndrome, Pruritis, Urticaria, Bullous eruptions and Hair loss. In addition to this, ACEIs can also cause a number of mucocutaneous adverse medication reactions.
Methods: This case was collected from Medicine out-patient department as a part of Pharmacovigilance elective under the Department of Pharmacology, CMC Ludhiana, which is an ADR Monitoring Centre.
Conclusion: The patient had erythematous rashes typically over the forearms, Periorbital area and on the back which are arranged in the form of clusters and associated with Itching, urticaria and redness. The patient had an erythematous rash associated with urticaria and also responded to treatment with anti-histamines and corticosteroids, thereby angioedema and drug hypersensitivity can be excluded.
Keywords: ramipril; antihypertensive drugs; erythematous rashes; cutaneous adverse drug reactions
Introduction
Hypertension, or high blood pressure, is a persistent increase in blood pressure above the normal range of 120/80 mm Hg [1]. It’s a major cause of premature death worldwide, affecting an estimated 1.28 billion adults aged 30-79 years. Hypertension can lead to serious health complications like heart disease, stroke, and kidney disease. Despite its prevalence, less than half of adults with hypertension are diagnosed and treated. Ramipril is one of the established anti- hypertensives and it belongs to the class of Angiotensin Convertase Enzyme Inhibitor (ACEI). It is approved for use in patients with the Hypertension as monotherapy or combination therapy [2]. In addition to its anti-hypertensive action, Ramipril enhanced endothelial function and raised Vascular Endothelial Growth Factor (VEGF) levels and the quantity of endothelial progenitor cell (EPC) which are contributing factors to cardiovascular disease [3].
Adverse drug reactions (ADR) such as dry cough and angioedema are a potential side effect of Ramipril that occasionally necessitate stopping the medication. The extent to which ACE inhibitors raise the risk of negative outcomes is yet unknown, despite the drug's extraordinary global rise in use [4]. In addition to this, ACEIs can cause a number of mucocutaneous adverse medication reactions, such as psoriasis, angioedema, pemphigus and pemphigoid. Alopecia, lichenoid drug eruptions, psoriasis, pseudolymphoma, photosensitivity, acquired brachial cutaneous dyschromatosis, eczematous response and pityriasis rosea are among the others, which are often moderate in intensity [5].
In ACE inhibitor-induced angioedema, there is no urticaria or itching, and the existence of urticaria indicates a distinct set of aetiologies [6]. So, was the scenario in this case, where the patient had an erythematous rash associated with urticaria, thereby angioedema and drug hypersensitivity can be excluded. Moreover, according to VigiAccess database, only 240 cases of Ramipril induced erythematous rash have been reported out of a total of 39,000 cases, which makes it a very rare occurrence [7]. It is important to note the such rare dermatological features for the clinicians. Hence, this case is worth reporting.
Case Report
This case involves a 57 years old patient with a history of hypertension since 07th Feb 2019. For the same she was started on Tab. Telmisartan 20mg HS which was changed to Tab. Losartan 25mg HS on 01st April 2019 to prevent further lowering and maintenance of baseline blood pressure. She is non-compliant to medications and took it in an on and off manner. Later, on 06th March 2024, she presented to OPD with the complaints of high blood pressure and was started on Tab. Ramipril at the dose of 1.25mg O.D. On 26th March 2024 she started experiencing Erythematous Rashes typically over the forearms, Periorbital area and on the back which are arranged in the form of clusters and associated with Itching, urticaria and redness. Due to sudden exaggerated form, she experienced severe discomfort and took Tab. Fexofenadine 120mg as an OD dose alongside the medication. On 28th March 2024 she stopped taking the drug (Ramipril). She was also given dexamethasone along with epinephrine and her symptoms ameliorated. The Naranjo’s score was 5 (probable) and the World Health Organization (WHO) Uppsala Monitoring Centre (UMC) causality assessment showed probable correlation with the current adverse event [8].
Discussion
WHO defines ADR as “a response to a medication that is noxious and unintended and occurs at doses normally used in man” [9]. Systems for reporting adverse drug events (ADEs) are essential for tracking and identifying drug safety indicators [10]. Considering the potential impact on the population’s morbidity and well-being, the adverse drug reactions have become major concern in public health [11]. It has been noted that among the anti-hypertensive drugs, ACE inhibitors are responsible for approximately half of the cutaneous drug reactions [12].
Ramipril is a pro drug which hydrolyzes inside the body to produce its active metabolite ramiprilat, which is achieved by removing the ester group from ramipril. It acts by inhibiting Angiotensin Convertase Enzyme and is highly efficacious antihypertensive due to its longer duration of action. It helps in reducing and maintaining satisfactory baseline control of blood pressure during long term treatment [13]. Ramipril is available in doses of 1.25 mg, 2.5 mg, 5 mg and 10 mg. However, the initial starting dose is 2.5 mg in majority of the cases [14].
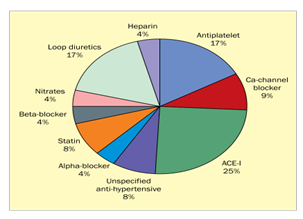
ACE Inhibitor induced angioedema has an occurrence of 0.7% of all treated patients and thereby a significant adverse event associated with drug use. However, in this case angioedema was ruled out as epicutaneous skin testing came out to be negative and moreover, the history and presenting symptoms did not give an indication of angioedema [15]. Ramipril induced erythematous rash has been rarely reported. Since ACE inhibitors like ramipril can cause acantholysis of epidermal cells in vitro, medications with ester groups may be implicated in causing rash, although the exact mechanism is still unclear. Although, one hypothesis postulates that the majority of ACE inhibitor-induced adverse dermatological responses are caused by non-immunological processes [16]. Another study done by Paulina et.al, reported that among all anti-hypertensive drugs, 25 % of all dermatological adverse effects are due to ACE Inhibitors in particular Ramipril [17]. This is also depicted in Figure 1.
Figure 1: Percentage of dermatological adverse events with anti-hypertensive drugs.
Despite this high incidence of dermatological side effects associated with ACE inhibitors, very few cases of erythematous rash have been reported as per literature search. This patient had a presentation of erythematous rash with urticaria which responded to treatment with anti-histamines and corticosteroids, thereby ruling out angioedema. Hence, this case is worth reporting and would add to the already significant list of dermatological side effects associated with ACE inhibitors and in particularly Ramipril.
Conclusion
Ramipril is being used as a monotherapy or combination therapy for management of Hypertension. Fewer studies from India have been shown to have such an adverse reaction which are either unreported or misdiagnosed. Hence, this case study is rare and worth reporting to raise awareness for the same in the general population.
Financial Support and Sponsorship
Nil
Conflict Interest
There is no conflict of interest.
Consent
Informed consent has been obtained for publication of this Case report.
References
- Sowmyashree, K., Vinutha, S., Venkatesh, K. G., Prajwal Kumar, Y. K., Bharathi, D. R., & Chandan, K. (2023). Title of the article. International Journal of Current Innovations in Advanced Research, 6(1):17.
Publisher | Google Scholor - World Health Organization. (2024). Hypertension.
Publisher | Google Scholor - Oliveira, A. C., Arismendi, M. I., Machado, L. S., & Sato, E. I. (2022). Ramipril improves endothelial function and increases the number of endothelial progenitor cells in patients with systemic lupus erythematosus. Journal of Clinical Rheumatology, 28(7):349-353.
Publisher | Google Scholor - Na Takuathung, M., Sakuludomkan, W., Khatsri, R., Dukaew, N., Kraivisitkul, N., Ahmadmusa, B., et al. (2022). Adverse effects of angiotensin-converting enzyme inhibitors in humans: A systematic review and meta-analysis of 378 randomized controlled trials. International Journal of Environmental Research and Public Health, 19(14):8373.
Publisher | Google Scholor - Lo, Y., & Tsai, T. F. (2022). Angiotensin converting enzyme and angiotensin converting enzyme inhibitors in dermatology: A narrative review. Expert Review of Clinical Pharmacology, 15(1):33-42.
Publisher | Google Scholor - Guyer, A. C., & Banerji, A. (2015). ACE inhibitor-induced angioedema.
Publisher | Google Scholor - VigiAccess. (2015). World Health Organization.
Publisher | Google Scholor - Naranjo, C. A., Busto, U., Sellers, E. M., Sandor, P., Ruiz, I., Roberts, E. A., et al. (1981). A method for estimating the probability of adverse drug reactions. Clinical Pharmacology & Therapeutics, 30:239-245.
Publisher | Google Scholor - Siddhu, C. K., Joseph, G., Bhatti, N., & Badyal, D. (2023). Paracetamol induced facial puffiness: An uncommon case report. NJPT, 1(3):170–172.
Publisher | Google Scholor - Lau, E. Y., Cragg, A., Small, S. S., Butcher, K., & Hohl, C. M. (2024). Characterizing and comparing adverse drug events documented in 2 spontaneous reporting systems in the Lower Mainland of British Columbia, Canada: A retrospective observational study. JMIR Human Factors, 11:e52495.
Publisher | Google Scholor - Rahmi Marliyeni, et al. (2021). Adverse drug reactions angioedema of ACE inhibitor: A review. IOSR Journal of Pharmacy and Biological Sciences, 16(1):12-20.
Publisher | Google Scholor - Ranugha, P. S., & Betkerur, J. B. (2018). Antihypertensives in dermatology part II: Cutaneous adverse reactions to antihypertensives. Indian Journal of Dermatology, Venereology and Leprology, 84:137.
Publisher | Google Scholor - Hermann, R., Gundlach, K., & Seiler, D. (2021). Mechanistic considerations about an unexpected ramipril drug-drug interaction in the development of a triple fixed-dose combination product containing ramipril, amlodipine, and atorvastatin. Clinical Pharmacology & Drug Development.
Publisher | Google Scholor - Heidenreich, P. A., Bozkurt, B., Aguilar, D., Allen, L. A., Byun, et al. (2022). 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Journal of the American College of Cardiology, 79(17):e263-e421.
Publisher | Google Scholor - Montinaro, V., & Cicardi, M. (2020). ACE inhibitor-mediated angioedema. International Immunopharmacology, 78:106081.
Publisher | Google Scholor - Agrawal, P., Sompura, S., Yadav, S. C., & Singh, A. K. (2013). Ramipril-induced bullous drug eruptions. Journal of Indian Academy of Clinical Medicine, 14(2).
Publisher | Google Scholor - Flis, P., Mehrholz, D., Barańska-Rybak, W., & Nowicki, R. (2016). Dermatological adverse effects of heart medicine: A retrospective study of patients in a dermatological ward in Gdansk between 2004-2013 and review of the literature. Folia Cardiologica, 11(3):186-193.
Publisher | Google Scholor