Research Article
Quantitative Analysis of EEG Signal in Drug-Resistant Juvenile Myoclonic Epilepsy
1 Department of Molecular and Clinical Pharmacology, Institute of Translational Medicine, Clinical Sciences Centre for Research and Education, University of Liverpool, UK.
2 Department of Neurophysiology, The Walton Centre, Liverpool, UK.
*Corresponding Author: Mariangela Panebianco, Department of Molecular and Clinical Pharmacology, Institute of Translational Medicine, University of Liverpool, Clinical Sciences Centre for Research and Education, Lower Lane, Liverpool, United Kingdom.
Citation: Panebianco M, Marson A, Radhika M. (2023). Quantitative Analysis of EEG Signal in Drug-Resistant Juvenile Myoclonic Epilepsy. Journal of Brain Research and Neurology, BRS Publishers. 1(1); DOI: 10.59657/2992-9768.brs.23.004
Copyright: © 2023 Mariangela Panebianco, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: February 09, 2023 | Accepted: February 23, 2023 | Published: March 03, 2023
Abstract
Juvenile Myoclonic Epilepsy (JME) is a genetic generalised epilepsy syndromes characterized by myoclonic jerks mostly in the morning, tonic-clonic seizures and absence seizures. About 30% of people with JME continue to have seizures despite treatment with antiepileptic drugs. Quantitative EEG (qEEG) is a computer analysis of the electroencephalography. The aim of this study was to compare qualitative vs quantitative EEG, quantitatively analyse background EEG activity in two different physiological states and the correlation with cognitive performances in patients with refractory JME. Inclusion criteria were: drug-resistant JME and aged between 14 and 65 years. The recorded ambulatory EEG data was subjected to visual and quantitative analysis and chosed two samples of 20-60 second duration per patient, morning sample (MS) and evening sample (ES). Our study cohort consisted of 19 patients with refractory JME, 6 females and 13 males, with a mean age of 29.8 years. EEG visual analysis of background activity revealed inter-ictal abnormalities in all patients localized to left frontal region. When we compared dominant rhythm in standard EEG with dominant rhythm in qEEG we have found that is <50% observer agreement. Agreement occurred in samples dominated by increased amount of theta and beta in both visual and qEEG. With spectral analysis there was no statistically significant difference of global absolute power (AP) in all the bands between MS and ES groups; the same result was found using Z scores. We have found an increase in mean AP Z scores delta, theta and beta in MS group and theta and beta in ES group, which was more evident in left frontal region. In addition, an increase theta/alpha index > 1.50 is associated with a decrease in cognitive IQ scores.
Keywords: refractory juvenile mioclonic epilepsy; quantitative EEG; spectral analysis; psysiological states; cognitive scores
Introduction
Juvenile Myoclonic Epilepsy (JME) is a common idiopathic generalised epileptic syndrome that occurs in about 5-11% of all epileptic syndrome. JME has a prevalence of 0.5 to 1.0 per 1000 with age of onset between 12 and 18 years [1]. The genetic generalised epilepsies (GGEs) are a group of epilepsy syndromes with a non-focal mechanism of seizure onset and no identifiable cause other than a proven or assumed genetic predisposition [2]. GGE constitute about 20% of all epilepsies, and include childhood absences epilepsy, juvenile absences and tonic clonic seizures on awakening. JME is characterized by myoclonic jerks mostly in the morning and tonic-clonic seizures (TCS) and around one third of people will also have absence seizures [3]. In JME deficits in executive and social functioning are common finding and related to impaired frontal lobe function. Some patients with JME have a family history of disease in variable ranges but for most people with JME the genes responsible have not yet been identified [4]. About 30% of people with JME continue to have seizures despite treatment with two or more antiepileptic drugs (refractory or drug resistant JME).
The electroencephalogram (EEG) is the most important method to diagnose epilepsy. In clinical settings, it is evaluated by experts who identify patterns visually [5]. The main EEG features of GGE are bilateral, synchronous, symmetric and generalised spike-wave-complex, and there are circadian variations of generalised epileptiform discharges. However, atypical epileptiform discharges are also commonly seen in GGE. Typical EEG features of JME consist of generalized discharges of single or multiple spike and slow-wave of frequency of 3-5 Hz, often with frontal-central accentuation, with normal background activity, although occasional complexes as slow as 2 Hz or as fast as 7 Hz may be evident. In addition, a detailed study of EEGs of patients with an unequivocal diagnosis of JME showed a high prevalence of focal EEG abnormalities. Focal EEG anomalies are evident in 15-40% of the patients such as focal slow waves, spikes and sharp waves and focal onset of the generalised discharge [6].
Quantitative EEG (qEEG) is a most recent type of EEG analysis that involves recording digital EEG signals which are processed, transformed and analyzed using complex mathematical algorithgms [7], and allows to obtain more precise and consistent results [8]. qEEG has been used in basic science and clinical studies of a range of neurological conditions including brain injury, epilepsy, sleep disorders, as well as in studies of consciousness and brain function [9, 10]. In clinical studies, EEG data from patients with a specific condition are usually compared with a reference database, usually of people without the condition in order to identify abnormalities associated with the condition. qEEG is a powerful method to evaluate the frequency composition of EEG activity [11, 12], and compared to the normal database [13]. Quantitative EEG techniques include frequency analysis (spectral analysis) and other analytic techniques [14, 15]. Each can be done on standard EEG in various physiological states or in conjunction with sensory stimulation [16, 17]. Several types of displays are available, including topographic mapping of scalp electrical activity. Analysis of data must take into account age, gender, state of alertness, medications and other factors [18, 19].
Most studies using qEEG in GGE have compared inter-ictal background quantitative EEG measures in generalized epilepsies with normal controls [20, 21], and few studies have specifically studied patients with JME as a separate group [8, 22]. Previous authors hypothesise that GGE frequency profiles reflect widespread cortical dysfunction common to all GGE syndromes [23]. In some cases of GGE, visual analysis of background EEG activity has been described as normal, except for some degrees of intermittent theta activity in patients with poor seizure control or in cases with antiepileptic polytherapy [24]. Statistically significant higher power (global α and θ, frontal α and θ, left temporal θ, right occipital α, δ and γ1, and central δ, θ, α, β, and γ2) was found in JME patients when compared to healthy controls [22]. Another recent study analysed background qEEG activity of patients with JME with and without antiepileptic drugs. It has found an increase in AP delta, alpha and beta bands, which is more evident in fronto-parietal regions [8]. To data, no studies assessed qEEG in refractory JME and/or correlated different quantitative EEG measures in several physiological states that typically influence the expressione of the seizures in these patients.
Objectives
We conducted this study with the aim of comparing qualitative vs quantitative EEG in adult subjects with drug-resistant JME. In addition, we compared quantitatively analyse background EEG activity in two different physicological states: morning and evening.
Methods
Population
The patients for our study are recruited from ReJuMEC (Refractory Juvenile Myoclonic Epilepsy Cohort) that was funded by the Medical Research Council in the UK. Inclusion criteria in ReJuMEC were: all the participants had JME [25] and were aged between 14 and 65 years.
Patients were recruited to ReJuMEC if they had a clinical diagnosis of drug-resistant JME including: 1. generalized seizures with myoclonic jerks with or without absence seizures and TCS; 2. no evidence of focal neurological or intellectual deficit; 3. refractory to treatment, specifying: the participants failed to achieve seizure control with sodium valproate at a dose of 1000 mg/day over a minimum period of 3 months; 4. the participants had an average of 4 days with myoclonic seizures per month over the past 3 months or an average of 4 days with absence seizures per month over the past 3 months. The main ReJuMEC study objectives were: to characterize the participants using EEG and neuropsychology testing; to obtain DNA samples for use in research investigating the genetics of epilepsy and its response to treatment. Following informed consent, detailed clinical and family history was taken, a DNA sample was taken, ambulatory EEG recording and neuropsychometric testing were undertaken.
EEG protocol
Patients enrolled into ReJuMEC trial underwent ambulatory EEG recording that included 24-48 hours of recording without video-recording using the Xltek System. Amplifier characteristics were: 10,000 dB gain, low cut filters at 0.05 Hz and high filters at 70 Hz. The recording was bipolar consisting of 19 electrodes in the standard International 10/20 System. The impedance was under 5 KΩ in all electrodes. The sampling frequency was 256 Hz.
Spectral EEG analysis
The recorded ambulatory EEG data was analyzed by two clinical neurophysiologists separately, in two different times, who selected appropriate artefact free EEG epochs from the awake state with eyes closed. They then reviewed the selected epochs and chose two samples of 60 second duration per patient, morning sample (MS) and evening sample (ES). The MS was taken from 2 hours to 5 after waking and the ES was taken at least 8 hours after waking. Artefact such as muscle/movement artefact, sweat artefact, drowsiness (identified by lateral eye movements or vertex sharp waves) and electrical interference were excluded. Each sample was subjected to visual and quantitative analysis by the same neurophysiologists who were blinded to patient selection. The samples were converted to EDF and exported to the qEEGNeuroGuide software. The quantitative analysis of background EEG activity was carried out with Fast Fourier Transform (FFT) [26]. EEG epochs were re-computed against common average reference. Spectral power, expressed in µV2, was calculated using Welch’s averaged periodogram method [27]. Frequencies between 0.5 and 30 Hz were divided into delta (0.5-4 Hz), theta (4-7 Hz), alpha (8-12 Hz), and beta (13-30 Hz) bands and analysed [26]. Spectral power was averaged region-wise (right and left frontal, parietal, temporal and occipital, and central).
The following parameters were assessed for spectral/quantitative analysis for each sample:
a) Dominant rhythm in standard EEG (sEEG) via qualitative visual analysis was compared to dominant rhythm in quantitative EEG (qEEG) samples.
b) The global absolute power was calculated for each frequency for each sample.
c) These parameters of global absolute power were also compared with Z scores, available on the software. These Z-values for each variable and band were obtained by comparison with population (American population) parameters based on the age-dependent regression function. Values outside the interval -1.96 to 1.96 were considered abnormal.
d) The mean global absolute power of each frequency was calculated in each sample within our cohort.
e) The mean absolute power Z scores per frequency per each lead were also calculated in morning and evening samples.
f) Theta/alpha index for each sample was calculated and then compared between the two groups. The cut off for the Theta/Alpha index was considered to be 1.50; values greater than 1.50 were considered abnormal.
g) Theta/alpha index was compared to Cognitive IQ Scores of 12 participants who underwent neuropsychological testing (WAIS-III test) during ReJuMEC study.
Results
Clinical characteristics
Our study cohort consisted of 19 patients with rJME, 6 females and 13 males, all Caucasian, with a mean age of 29.8 years, and an age range between 16 and 60 years. The mean age of onset of epilepsy was 12.8 years with a mean epilepsy duration of 17.4 years. All patients presented with myoclonic seizures, 16 patients had absences and 16 tonic-clonic seizures (TCS). Myoclonic seizures were always present in the first hour after awakening. The patients had normal neurological examination and characteristic paroxysmal generalized activity with polyspike-wave complexes (PSWC) in EEG recordings. All patients had failed to achieve seizure control on valproate and were taking several medication at the time the EEG was undertaken (see Table 1).
Table 1. Clinical characteristics of all the samples
| Number of partecipants | 19 |
| Gender n (%) | |
| Male | 6(31.6 %) |
| Female | 13(68.4 %) |
| Age (y) | |
| Mean ± SD | 29.8 ± 12.6 |
| Range | 16 - 60 |
| Onset of epilepsy (y) ± SD | 12.8 ± 2.8 |
| Years of seizure disorders ± SD | 17.4 ± 13.1 |
| Seizure types n (%) | |
| Myoclonic | 19 (100 %) |
| Absence | 16 (84.2 %) |
| TCS | 16 (84.2 %) |
| Treatment n (%) | |
| Monotherapy | 5(26.3 %) |
| Politherapy | 14 (73.7%) |
| AEDs (n) | |
| LTG | 6 |
| TPM | 9 |
| VPA | 7 |
| LEV | 9 |
| ZNS | 3 |
| CLB | 5 |
Spectral EEG analysis
We selected 38 samples; 19 morning samples (MS) and 19 evening samples (ES).
Qualitative versus quantitative assessment of dominant rhythm
We found correlation between MS and ES samples in 8 out of 19 samples (42% agreement; see Table 2). This is considered to be a fair agreement in both groups using Cohen’s Kappa coefficient (=0.261); 4.1% agreement occurred by chance.
Table 2. Comparison between dominant rhythm in standard EEG and dominant rhythm in quantitative EEG
| Morning Sample | Evening Sample | ||
| Visual Analysis Standard EEG | Quantitative analysis qEEG | Visual Analysis Standard EEG | Quantitative analysis qEEG |
| Beta | Delta | Beta * | Beta * |
| Theta * | Theta * | Theta | Delta |
| Theta * | Theta * | Beta * | Beta * |
| Theta | Alpha | Beta | Delta |
| Theta | Delta | Theta | Delta |
| Beta * | Beta * | Beta * | Beta * |
| Theta | Delta | Beta * | Beta * |
| Beta * | Beta * | Beta * | Beta * |
| Theta | Delta | Theta * | Theta * |
| Beta * | Beta * | Theta | Beta |
| Beta * | Beta * | Beta * | Beta * |
| Theta | Delta | Theta | Delta |
| Beta | Delta | Theta | Delta |
| Beta | Delta | Theta | Beta |
| Beta * | Beta * | Theta | Delta |
| Beta | Delta | Beta | Delta |
| Beta * | Beta * | Theta | Delta |
| Theta | Delta | Beta * | Beta * |
| Theta | Delta | Theta | Delta |
Global absolute power of morning versus evening samples
We found a predominance for theta and delta frequencies in the morning samples but this was not found to be statistically significant (p>0.05) (see Table 3).
Table 3. Global Absolute Power Z scores for each sample in two the groups
| Delta MS | Theta MS | Alpha MS | Beta MS | Delta ES | Theta ES | Alpha ES | Beta ES | ||
| 1 | 1.05 | 0.43 | -0.46 | 1.11 | 0.75 | 0.18 | -0.41 | 1.48 | |
| 2 | 2.01 | 1.99 | 0.37 | 0.95 | 0.71 | 1.17 | 0.07 | 1.33 | |
| 3 | 0.26 | -0.24 | -0.88 | 0.13 | 0.23 | 0.01 | -0.86 | 0.67 | |
| 4 | 0.14 | 1.84 | 0.53 | -0.42 | 1.12 | 1.23 | -0.37 | 0.88 | |
| 5 | 1.32 | 0.06 | 0.11 | 1.60 | 0.79 | 0.05 | 0.42 | 1.75 | |
| 6 | 0.38 | 0.46 | -0.76 | -0.79 | 0.22 | 0.92 | -0.35 | 0.01 | |
| 7 | 0.41 | 0.30 | 0.06 | 1.70 | 8.47 | 0.57 | 0.14 | 0.83 | |
| 8 | 1.43 | 0.88 | 0.68 | 1.21 | -0.27 | -0.27 | -0.57 | 1.00 | |
| 9 | 0.73 | 2.46 | 0.47 | -0.23 | 0.02 | 0.62 | 0.02 | 0.55 | |
| 10 | -0.68 | 0.14 | 0.37 | 0.64 | 0.94 | 0.37 | 0.25 | 0.41 | |
| 11 | 0.49 | 1.15 | 0.63 | 1.20 | 0.52 | 1.10 | 0.66 | 1.22 | |
| 12 | -0.44 | -0.43 | -0.99 | -1.18 | -0.53 | -0.74 | -1.41 | -0.72 | |
| 13 | 1.93 | 1.80 | -0.70 | -0.34 | 1.94 | 2.40 | 0.35 | -0.51 | |
| 14 | 0.33 | 0.83 | -0.87 | 0.54 | -0.42 | -0.20 | -1.02 | 0.02 | |
| 15 | 1.71 | 3.29 | 0.80 | 0.95 | 0.63 | 1.24 | 0.15 | -0.39 | |
| 16 | -0.45 | -0.15 | 0.42 | 1.80 | 0.31 | -0.16 | 0.09 | 1.38 | |
| 17 | 3.81 | 3.47 | 1.64 | 1.34 | 2.64 | 3.61 | 1.83 | 1.45 | |
| 18 | 1.14 | -0.08 | -0.92 | 0.18 | 0.98 | 0.48 | 0.01 | 0.37 | |
| 19 | 1.07 | 1.23 | 0.06 | 1.11 | 1.44 | 1.25 | 0.30 | 1.03 |
Abbreviations: Pt: patient; MS: morning sample; ES: evening sample.
Z scores global absolute power of morning versus evening samples
We reported the results of this comparison in the table 3. Global AP showed highest values in delta band in the ES group, but no statistically significant differences were seen (p>0.05) between two groups (see Table 4).
Table 4. Student’s t-test
Global Absolute Power
| Delta | Theta | Alpha | Beta | |
| p-value | 0.549 | 0.253 | 0.596 | 0.861 |
| t-value | 0.61 | 1.16 | 0.53 | -0.18 |
Global Absolute Power Z scores
| Delta | Theta | Alpha | Beta | |
| p-value | 0.592 | 0.292 | 0.113 | 0.455 |
| t-value | 0.54 | 1.07 | 1.63 | 0.76 |
Global mean absolute power of morning versus evening samples
The analysis showed that the global mean AP was highest within delta, theta and beta frequency bands with a significant increase in delta and theta in MS group (see Table 5).
Table 5. Global Mean Absolute Power for each frequency in two the groups
| Morning Sample | Evening Sample | |
| Delta | 15.50 | 11.77 |
| Theta | 9.29 | 7.08 |
| Alpha | 6.77 | 6.12 |
| Beta | 10.54 | 10.33 |
Mean AP Z scores of mornings versus evening samples
We found that mean AP of delta band was the highest power frequency in left frontal leads in MS group and mean AP of beta band was the highest power frequency in left frontal lead in ES group (see Table 6).
Table 6. Mean Absolute Power Z scores per frequency per region in all the samples
| Delta MS | ES | Theta MS | ES | Alpha MS | ES | Beta MS | ES | Total MS | ES | |
| FP2-F8 | 1.43 | 1.30 | 1.51 | 1.16 | 0.79 | 0.64 | 1.32 | 1.21 | 5.05 | 4.31 |
| F8-T4 | 0.48 | 0.46 | 1.01 | 0.57 | 0.11 | 0.05 | 0.80 | 0.73 | 2.40 | 1.81 |
| T4-T6 | 0.52 | 0.27 | 0.62 | 0.29 | -0.40 | -0.47 | 0.32 | 0.44 | 0.59 | 0.53 |
| T6-O2 | 0.50 | 0.29 | 0.63 | 0.38 | -0.55 | -0.55 | -0.36 | 0.04 | 0.22 | 0.16 |
| FP2-F4 | 1.23 | 0.94 | 1.22 | 0.81 | 0.47 | 0.35 | 1.45 | 1.38 | 4.37 | 3.48 |
| F4-C4 | 1.22 | 0.67 | 1.32 | 0.79 | 0.01 | -0.07 | 0.59 | 0.74 | 3.14 | 2.13 |
| C4-P4 | 0.73 | 0.33 | 0.62 | 0.32 | -0.43 | -0.54 | 0.03 | 0.06 | 0.95 | 0.17 |
| P4-O2 | 1.18 | 0.69 | 0.97 | 0.74 | -0.36 | -0.38 | 0.08 | 0.31 | 1.87 | 1.36 |
| FP1-F7 | 1.99 | 1.82 | 2.27 | 2.00 | 1.35 | 1.22 | 2.09 | 2.02 | 7.70 | 7.06 |
| F7-T3 | 0.22 | 0.25 | 0.72 | 0.55 | 0.02 | 0.13 | 0.79 | 0.83 | 1.75 | 1.76 |
| T3-T5 | 0.49 | 0.43 | 0.68 | 0.48 | -0.25 | -0.28 | 0.58 | 0.61 | 1.50 | 1.24 |
| T5-O1 | 0.46 | 0.29 | 0.68 | 0.42 | -0.40 | -0.50 | -0.20 | -0.15 | 0.54 | 0.06 |
| FP1-F3 | 1.52 | 1.54 | 1.78 | 1.56 | 0.96 | 0.90 | 1.67 | 1.69 | 5.93 | 5.69 |
| F3-C3 | 0.76 | 0.58 | 1.03 | 0.72 | -0.04 | -0.09 | 0.56 | 0.67 | 2.31 | 1.88 |
| C3-P3 | 0.29 | -0.06 | 0.33 | 0.08 | -0.67 | -0.67 | -0.26 | -0.17 | -0.31 | -0.82 |
| P3-O1 | 1.15 | 0.77 | 0.97 | 0.77 | -0.31 | -0.33 | 0.21 | 0.29 | 2.02 | 1.50 |
| Total | 14.17 | 10.57 | 16.36 | 11.64 | 0.30 | -0.59 | 9.00 | 10.70 | 40.03 | 32.32 |
Abbreviations: MS: morning sample; ES: evening sample.
Theta/alpha index of morning versus evening samples
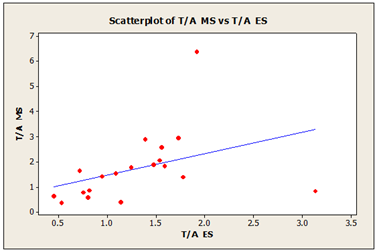
As shown in the scatter plot diagram (Figure 1) a positive correlation was seen between theta-alpha index in MS and ES with R-squared value of 32.3%. This is influenced by the increased of theta power in the samples.
Theta/alpha index versus cognitive scores
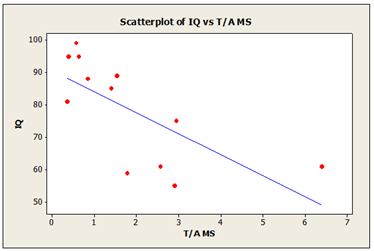
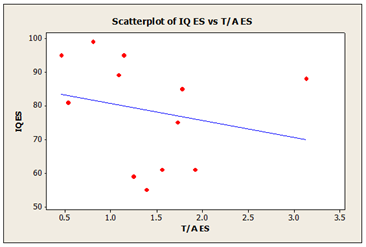
In addition, we have compared global theta/alpha index to cognitive IQ score for 12 participants, who underwent to neuropsychological testing (WAIS-III test) during ReJuMEC study. High IQ scores was associated with small theta-alpha index and low IQ scores was associated with high theta-alpha index, as shown in the same table 7. In the MS group the scatter plot diagrams (Figure 2) shows a negative correlation between IQ scores and theta-alpha index with R-squared value of 58.7%. The scatter plot diagrams (Figure 3) shows a weak correlation in ES group between IQ scores and theta-alpha index with R-squared value of 12%.
Table 7. Theta/alpha index and cognitive IQ scores of study groups
| T/A Index MS | T/A Index ES | Cognitive IQ scores | |
| Pt | |||
| 1 | 1.54 | 1.09 | 89 |
| 2 | 1.89 | 1.48 | - |
| 3 | 2.06 | 1.54 | - |
| 4 | 0.84 | 3.13 | 88 |
| 5 | 0.63 | 0.46 | 95 |
| 6 | 1.82 | 1.59 | - |
| 7 | 0.79 | 0.76 | - |
| 8 | 0.40 | 1.14 | 95 |
| 9 | 2.57 | 1.56 | 61 |
| 10 | 0.57 | 0.81 | 99 |
| 11 | 0.85 | 0.82 | - |
| 12 | 1.40 | 1.78 | 85 |
| 13 | 6.39 | 1.92 | 61 |
| 14 | 2.95 | 1.73 | 75 |
| 15 | 2.90 | 1.39 | 55 |
| 16 | 0.37 | 0.54 | 81 |
| 17 | 1.78 | 1.25 | 59 |
| 18 | 1.65 | 0.72 | - |
| 19 | 1.42 | 0.95 | - |
| Mean | 1.70 | 1.20 |
Abbreviations: MS: morning sample; ES: evening sample; IQ: Intelligence Quotient; Pt: patient.
Figure 1: Theta/Alpha index in morning sample (MS) and evening sample (ES) R-Sq=32.3%
Figure 2: Theta-Alpha index in morning sample (MS) and cognitive IQ scores R-Sq=58.7%
Figure 3: Theta-Alpha index in evening sample (ES) and cognitive IQ scores R-Sq=12%
Discussion
The advantage of qEEG over visual analysis of EEG is evident, and this has been observed in neurological and psychiatric diseases [28]. In this study a key advantage we had was prolonged ambulatory EEG data. This gave us the opportunity to compare various physiological states of the patients, i.e. morning and evening.
It is documented that AP findings in qEEG were interpreted as enhanced synchronization of different neuronal population in the 0.5-12.0 Hz frequency range together with the tendency of decreasing synchrony in faster (12.5-32.0 Hz) frequencies [29].
Neuronal network hypersynchronization is a fundamental mechanism in IGE. The delta band range lies within the same polyspike-wave complexes range characteristic of JME. In fact, the increase in AP delta is common to all IGE syndromes sharing the same psysiophatological mechanism [30].
In previous studies patients with JME have shown an increase in AP delta, alpha and beta bands, which is more evident in fronto-parietal regions [8, 31]. In addition, left frontal and central theta/alpha index has significant correlation with the presence of cognitive disturbance [32].
In our study standard EEG visual analysis of background activity revealed inter-ictal abnormalities in all patients, in the morning and evening samples. This is commonly seen in JME patients with poor seizure control especially in cases with antiepileptic polytherapy. During visual analysis the alterations found in background EEG activity in patients with JME were localized to left frontal region. When we compared dominant rhythm in standard EEG with dominant rhythm in quantitative EEG we have found that is <50>
With spectral analysis for each sample the global absolute power in all bands showed not statistical significant differences between MS and ES groups. In brief, when MS and ES groups were compared, there was no statistically significant difference of global power in all the bands; the same result was found using Z scores. A positive correlation is seen in theta and beta bands between the two groups, while alpha and delta bands showed weakly positive correlation. Theta is increased in the MS but not significantly. We think that this lack of statistical difference is due to our relatively small sample size.
In patients with refractory JME, qEEG values reveal abnormal Z scores in a high number of samples, when compared with normal population parameters based on the age-dependent regression function. Abnormal Z-scores in AP and in RP were present in all bands, measures and leads, with predominance in MS group.
In the analysis of our cohort, all the samples were characterised by increased global mean absolute power in delta and theta bands more evident in MS group and in beta band more evident in ES group. In addition, there was an increase in global absolute power in theta and beta bands when comparing morning and evening sample. Possibilities that might explain this include the patients’ antiepileptic medications and their dosage and possibly fragmented sleep, although all the morning samples were obtained 2 hours after full wakening.
In addition, there is an increase in mean AP Z scores delta, theta and beta in MS group and theta and beta in ES group, which is more evident in left frontal region. This result is in agreement with previous studies on JME. Delta, theta and beta bands are increased and are abnormal when compared to Z scores. Alpha band was within normal limits compared to Z scores and was the same in MS and ES. However, these changes were not statistically significant most probably due to our small sample size.
A positive correlation is seen between theta/alpha index in MS and ES group. We have found that increase theta/alpha index > 1.50 is associated with a decrease in cognitive IQ scores. This result confirms that theta/alpha index can be used as marker for cognitive deficit. The correlation between global theta/alpha index with Cognitive IQ scores is influenced by increased theta power.
Conclusion
Our conclusion is that these findings suggest that the centre of interictal epileptogenic activity is in left frontal region and that is not significant difference between physiological states, morning or evening of the patients with rJME. This rJME profiles reflect widespread cortical dysfunction essentially common to all the investigated IGE syndromes. The certainty of our conclusions is probably limited by small sample size and by polytherapy and by AEDs as VPA that is known to influence EEG power.
Declarations
Acknowledgements
The authors are grateful to Prof. Gus Baker coordinated the IQ assessments, Dr. Graeme Sills set up ReJuMec Study and Dr.Joun Paul Leach recorded of the EEGs.
Funding
This research received no external funding.
Conflict of Interest
The authors declare no conflict of interest.
References
- Commission on Classification and Terminology of the International League Against Epilepsy. (1989). Proposal for revised classification of epilepsies and epileptic syndromes.. Epilepsia, 30(4):389-399.
Publisher | Google Scholor - Panayiotopoulos CP. (2005). Idiopathic generalized epilepsies: a review and modern approach. Epilepsia, 46(S9):1-6.
Publisher | Google Scholor - Genton P, Gelisse P. (2001). Juvenile Myoclonic Epilepsy. Arch Neurol, 58(9):1487-1490.
Publisher | Google Scholor - Zifkin B, Andermann E, Andermann F. (2005). Mechanisms, genetics, and pathogenesis of juvenile myoclonic epilepsy. Curr Opin Neurol. 18(2):147-153.
Publisher | Google Scholor - Holler Y, Nardone R. (2021). Quantitative EEG biomarkers for epilepsy and their relation to chemical biomarkers. Adv Clin Chem, 102:271-336.
Publisher | Google Scholor - Aliberti V, Grünewald RA, Panayiotopoulos CP, Chroni E. (1994). Focal electro encephalo graphic abnormalities in juvenile myoclonic epilepsy. Epilepsia, 35(2):297-301.
Publisher | Google Scholor - Livint Popa L, Dragos H, Pantelemon C, Verisezan Rosu O, Strilciuc S. (2020). The Role of Quantitative EEG in the Diagnosis of Neuropsychiatric Disorders. J Med Life, 13(1):8-15.
Publisher | Google Scholor - Santiago-Rodríguez E, Harmony T, Cárdenas-Morales L, Hernández A, Fernández-Bouzas A. (2008). Analysis of background EEG activity in patients with juvenile myoclonic epilepsy. Seizure, 17(5):437-445.
Publisher | Google Scholor - Thakor NV, Tong S. (2004). Advances in quantitative electroencephalogram analysis methods. Annu Rev Biomed Eng, 6:453-495.
Publisher | Google Scholor - Koberda J. (2012). Application of Quantitative Electroencephalography (QEEG) and Low Resolution Electro-Magnetic Tomography Analysis (LORETA) in General Neurology Practice for Detection and Localization of Epilepsy. Neurology, 78(S1):P01.050
Publisher | Google Scholor - Nuwer MR. (1988). Quantitative EEG: I. Techniques and problems of frequency analysis and topographic mapping. J Clin Neurophysiol, 5(1):1-43.
Publisher | Google Scholor - Nuwer MR. (1988). Quantitative EEG: II. Frequency analysis and topographic mapping in clinical settings. J Clin Neurophysiol. 5(1):45-85.
Publisher | Google Scholor - Lehnertz K, Andrzejak RG, Arnhold J, Kreuz T, Mormann F, Rieke C, Widman And G, Elger CE. (2001). Nonlinear EEG analysis in epilepsy: its possible use for interictal focus localization, seizure anticipation, and prevention. J Clin Neurophysiol. 18(3):209-222.
Publisher | Google Scholor - Díaz GF, Virués T, San Martín M, Ruiz M, Galán L, Paz L, Valdés P. (1998). Generalized background qEEG abnormalities in localized symptomatic epilepsy. Electroencephalogr Clin Neurophysiol. 106(6):501-507.
Publisher | Google Scholor - Gevins A. (1987). Overview of computer analysis. In: Gevins A., Remond A. (Eds). Methods of Analysis of Brain Electrical and Magnetic Signals. EEG Handbook, revised series, vol.1. Amsterdam: Elsevier.
Publisher | Google Scholor - Lehmann D. (1987). Principles of spatial analysis. In: Gevins A., Remon A. (Eds). Analysis of Electrical and Magnetic Signals. Handbook of Electroencephalography and Clinical Neurophysiology. Amsterdam: Elsevier, 1-46.
Publisher | Google Scholor - Gotman J. (1986). Computer analysis of EEG in epilepsy. In: Lopes Da Silva F.H., Storm Van Leeuwen W., Remond A. (Eds). Clinical application of Computer Analysis of EEG and other Neurophysiological Signals. Handbook of Electroencephalography and Clinical Neurophysiology, revised series, 2:171-204.
Publisher | Google Scholor - Numer MR. (1988). Frequency analysis and topographic mapping of EEG and evoked potentials in epilepsy, Electroencephalography and Clinical Neurophysiology, 69(2):118-126.
Publisher | Google Scholor - Nuwer MR, Lehmann D, Lopes da Silva F, Matsuoka S, Sutherling W, Vibert JF. (1994). IFCN guidelines for topographic and frequency analysis of EEGs and EPs. Report of an IFCN committee. International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol, 91(1):1-5.
Publisher | Google Scholor - Clemens B, Puskás S, Bessenyei M, Emri M, Spisák T, Koselák M, Hollódy K, Fogarasi A, Kondákor I, Füle K, Bense K, Fekete I. (2011). EEG functional connectivity of the intrahemispheric cortico-cortical network of idiopathic generalized epilepsy. Epilepsy Res, 96(1-2):11-23.
Publisher | Google Scholor - Miyauchi T, Endo K, Yamaguchi T, Hagimoto H. (1991). Computerized analysis of EEG background activity in epileptic patients. Epilepsia. 32(6):870-881.
Publisher | Google Scholor - Tikka SK, Goyal N, Umesh S, Nizamie SH. (2013). Juvenile myoclonic epilepsy: Clinical characteristics, standard and quantitative electroencephalography analyses. J Pediatr Neurosci, 8(2):97-103.
Publisher | Google Scholor - Clemens B, Szigeti G, Barta Z. (2000). EEG frequency profiles of idiopathic generalised epilepsy syndromes. Epilepsy Res. 42(2-3):105-115.
Publisher | Google Scholor - Clemens B, Bessenyei M, Piros P, Tóth M, Seress L, Kondákor I. (2007). Characteristic distribution of interictal brain electrical activity in idiopathic generalized epilepsy. Epilepsia. 48(5):941-949.
Publisher | Google Scholor - Commission on Classification and Terminology of the International League Against Epilepsy. (1989). Proposal for revised classification and terminology of epilepsies and epileptic syndromes. Epilepsia, 30(4):389-399.
Publisher | Google Scholor - Michel CM, Lehmann D, Henggeler B, Brandeis D. (1992). Localization of the sources of EEG delta, theta, alpha and beta frequency bands using the FFT dipole approximation. Electroencephalogr Clin Neurophysiol. 82(1):38-44.
Publisher | Google Scholor - Welch PD. (1967). The use of Fast Fourier Transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms, in IEEE Transactions on Audio and Electroacoustics, 15(2):70-73.
Publisher | Google Scholor - Chen, A.C.N., Rappelsberger, P. (1994). Brain and Human pain: Topographic EEG amplitude and coherence mapping. Brain Topogr, 7:129-140.
Publisher | Google Scholor - Muthuswamy J, Thakor NV. (1998). Spectral analysis methods for neurological signals. J Neurosci Methods, 83(1):1-14.
Publisher | Google Scholor - Gloor P. (1984). Electrophysiology of generalised epilepsy. In: Schwartzkroin PA, Wheal HV, editors. Electrophysiology of epilepsy. London: Academic Press, 107-136.
Publisher | Google Scholor - Thatcher RW, North D, Biver C. (2005). Evaluation and Validity of a LORETA Normative EEG Database. Clinical EEG and Neuroscience, 36(2):116-122.
Publisher | Google Scholor - Schmidt MT, Kanda PA, Basile LF, Lopes da Silva FH, Baratho R, Demario JL, Jorge MS, Nardi AE, Machado S, Ianof JN, Nitrini R, Anghinah R. (2013). Index of alpha/theta ratio of the electroencephalogram: a new marker for Alzheimer’s disease. Front Aging Neurosci 9(5):60.
Publisher | Google Scholor