Case report
Pneumonia before COVID-19 Pandemic Emerging with Accidental Brugada Syndrome and Thrombocytopenia-Serious Association with Good Outcome
Critical Care Unit, Kafr El-Bateekh Central Hospital, Damietta Health Affairs, Egyptian Ministry of Health, Damietta, Egypt.
*Corresponding Author: Yasser Mohammed Hassanain Elsayed, Critical Care Unit, Kafr El-Bateekh Central Hospital, Damietta Health Affairs, Egyptian Ministry of Health, Damietta, Egypt.
Citation: YMH Elsayed. (2023). Pneumonia before COVID-19 Pandemic Emerging with Accidental Brugada Syndrome and Thrombocytopenia- Serious Association with Good Outcome. International Clinical and Medical Case Reports, BRS Publishers. 2(1); DOI: 10.59657/2837-5998.brs.23.004
Copyright: © 2023 Yasser Mohammed Hassanain Elsayed, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: September 05, 2022 | Accepted: December 30, 2022 | Published: January 06, 2023
Abstract
Rationale: COVID-19 pneumonia emerged in Wuhan city in China on December 31, 2019. However, pneumonia is a serious infection cause. Brugada syndrome [BrS] is a genetic disorder with abnormal electrical activity within the heart. The syndrome carries a higher risk for ventricular fibrillation and sudden death. Thrombocytopenia may be associated with the severe acute respiratory syndrome. Probably Thrombocytopenia is a well-known marker of adverse outcomes in patients diagnosed with pneumonia, as decreased platelet count is linked to severe intravascular coagulation and severe sepsis. Patient concerns: A 50-year-old, bed-ridden smoker Egyptian married male patient was admitted to the intensive care unit with pneumonia and Brugada syndrome [BrS].
Diagnosis: Pneumonia before COVID-19 pandemic emerging with accidental Brugada syndrome and thrombocytopenia.
Interventions: Electrocardiography, chest CT scan, oxygenation, and arterial blood gas.
Outcomes: Electrocardiographic and clinical stabilization was the only result.
Lessons: The presence of mimic COVID-19 pneumonia about 1 year before emerge of the COVID-19 pandemic is interesting and stupendous. The association of Brugada syndrome, renal impairment, and thrombocytopenia in pneumonia are a constellation of serious risk factors.
Keywords: pneumonia; brugada syndrome; covid-19 pandemic emerging; thrombocytopenia; accidental; serious association
Introduction
Pneumonia is a prevalent and likely serious disease [1]. It is correlating to high mortality, particularly with associated co-morbidities. Ambiguous cases of pneumonia emerged in Wuhan city in China on December 31, 2019. On January 7, 2020. Coronavirus (2019-nCoV) was identified as a new causative agent and the disease was later named COVID-19 by the WHO [2]. Pneumonia is a lung infection that can vary from mild to severe illness affecting all ages [3]. Viruses, bacteria, and fungi are implicated cause in pneumonia. Influenza, respiratory syncytial virus (RSV), and SARS-CoV-2 (the virus that causes COVID-19) are detected as common causes of viral pneumonia. Pneumococcus pneumonia represents a frequent cause of bacterial pneumonia [3]. Brugada syndrome (BrS) is a genetic disorder with abnormal electrical activity within the heart. The syndrome carries a higher risk for ventricular fibrillation (VF) and sudden cardiac death (SCD) in a structurally normal heart [SHD] [4]. Three classes were described; Coved type (class I) has a coved type ST-segment elevation with ≥ 2 mm (0.2 mV) J-point elevation and a gradually descending ST-segment followed by a negative T-wave [4]. Thrombocytopenia is a well-known marker of adverse outcomes in patients diagnosed with pneumonia, as decreased platelet count is linked to severe intravascular coagulation and severe sepsis [4]. Platelets are considered a cornerstone in the process of hemostasis [1]. Immune thrombocytopenic purpura (ITP) is an isolated autoimmune thrombocytopenic disorder. Viruses such as HIV, MCV, EBV, parvovirus, rubella, measles, and coronavirus (COVID-19) infection are already identified as triggering factors for the autoimmune process [5]. ITP is characterized by isolated thrombocytopenia (PLT <100>6]. Autoimmune antibodies or immune complexes triggered by viral infection may represent a remarkable role in causing thrombocytopenia [5]. Normal platelet count in humans ranges from 150,000 to 450,000 c/ml. Platelet transfusion is essentially indicated to treat or prevent bleeding in thrombocytopenia or platelet function disorder. The Platelet transfusion threshold in bleeding is A. if <50>7].
Case Presentation
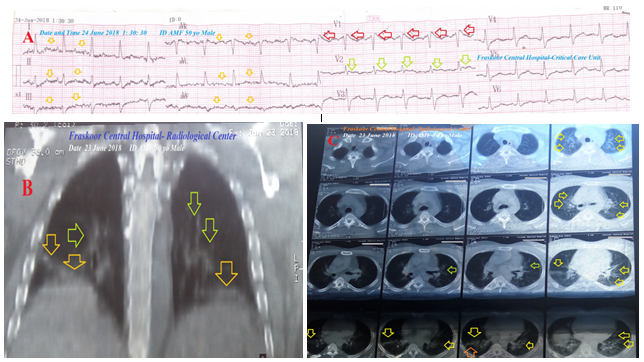
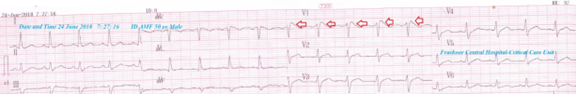
A 50-year-old married, bed-ridden, smoker, Egyptian, male patient was admitted to the intensive care unit (ICU) with tachypnea and palpitations. Fever, generalized body aches, hematuria, and fatigue were the associated symptoms. The patient is a currently heavy smoker (at least 20 cigarettes for about 20 years). He gave an old history of fall trauma from hill resulting in post-traumatic paraplegia 5 years ago. The patient gave a history of his night snoring and idiopathic familial sudden death. Upon general physical examination; generally, the patient was bed-ridden, immobilized, paraplegic, irritable, and distressed, with a regular rapid heart rate of 110 bpm, blood pressure of 110/70 mmHg, respiratory rate of 22 bpm, a temperature of 38.5 °C, and pulse oximeter of O2 saturation of 96%. No more relevant clinical data were noted during the clinical examination. Urgent initial ECG tracing was done on the presentation in the ICU showing a sinus tachycardia (VR;110), a Coving ST-segment sign of Brugada syndrome appears in V1 lead, and saddle-shape ST-segments elevation inV2 lead (Figure 1A). The patient was treated with O2 inhalation (100%, by nasal cannula, 5L/min), cefotaxime; (1 gm IV TID), IV of ampicillin with sulbactam vial (1.5 gm, TDS), and paracetamol (500 mg TID as needed). Platelets transfusion (10 ml/kg, with a transfusion rate of 2 ml/min) was added. The complete blood count (CBC); Hb was 11.9 g/dl, RBCs; 4.81*103/mm3, WBCs; 3000*103/mm3 (Neutrophils; 86 %, Lymphocytes: 9%, Monocytes; 4%, Eosinophils; 1% and Basophils 0%), Platelets; 22*103/mm3. Serum creatinine (4.3 mg/dl) and blood urea were high (192 mg/dl). RBS was normal (97 mg/dl). Ionized calcium was slightly low (0.91 mmol/L). Plasma sodium (138 mmol/L) and serum potassium were normal (4.2 mmol/L). SGPT (35 U/L) and SGOT were normal (26 U/L). Total bilirubin was normal (0.6 mg/dl). The prothrombin time test (PT, 23 sec), International Normalized Ratio (INR) (1.9) were high. D-dimer was high (1122 ng/ml). The troponin test was negative (0.03 ng/L). ABG showed respiratory alkalosis. Plain CXR film showing bilateral ground glass opacities and bilateral halo sign (Figure 1B). Chest CT without contrast (due to high creatinine) shows bilateral ground glass opacities, bilateral reversed halo sign, and right reversed halo sign (Figure 1C). Pneumonia before the COVID-19 pandemic emerging with accidental Brugada syndrome and thrombocytopenia was the most probable current diagnosis. An ECG tracing was done within 6 hours of the ICU admission showing NSR (of VR; 97) and Coving ST-segment sign of Brugada syndrome still appears in V1 lead (Figure 2). The patient was discharged on the third day after general and clinical stabilization. The patient was continued: ampicillin with sulbactam (625 mg, TID daily, for 7 days). The patient was advised for cardiovascular, pulmonary, renal, and hematological follow-up.
Figure 1A: An initial ECG tracing was done on the presentation in the ICU showing sinus tachycardia (of VR;110), Coving ST-segment sign of Brugada syndrome appears in V1 lead (red arrows), and saddle shape ST-segments elevation inV2 lead (lime arrows). There are tremor artifacts (orange arrows).
Figure 1B: Plain CXR film showing bilateral ground glass opacities (orange arrows) and bilateral halo sign (lime arrows).
Figure 1C: Chest CT scan film showing bilateral ground glass opacities (orange arrows), bilateral reversed halo sign (lime arrows), and right reversed halo sign (orange arrows).
Figure 2: An ECG tracing was done within 6 hours of the ICU admission showing NSR (of VR; 97), Coving ST-segment sign of Brugada syndrome appears in V1 lead (red arrows).
Discussion
Overview
- A 50-year-old, bed-ridden smoker Egyptian married male patient was admitted to the intensive care unit with pneumonia, Brugada syndrome, thrombocytopenia, and renal impairment.
- The primary objective for my case study was the presence of a patient who presented with pneumonia, Brugada syndrome, thrombocytopenia, and renal impairment involvement in the ICU.
- The secondary objective for my case study was the question; How did you manage the case at home?
- The presence of fever, tachypnea, sinus tachycardia, bilateral ground glass opacities, bilateral reversed halo sign, and right reversed halo sign in both chest CT and the plain film will strengthen the diagnosis of acute pneumonia.
- The associated sinus tachycardia with tachypnea and elevated D-dimer will be directed to acute pulmonary embolism but CT pulmonary angiogram (or CTPA) can't be done due to the presence of renal impairment.
- Leucopenia exists with viral pneumonia. But COVID-19 pandemic didn’t emerge at this time.
- History of patient night snoring, idiopathic familial sudden death, electrocardiographic evidence of a Coving ST-segment sign of Brugada syndrome appears in V1 lead, and saddle-shape ST-segments elevation inV2 lead is the indicator for the current Brugada syndrome.
- Right bundle branch block (RBBB) is considered in the differential diagnosis for Brugada syndrome. RBBB is mostly restricted to V1-V2 but with slurred S-wave in V5-V6.
- Hematuria with a severe reduction in platelet count is evidence of severe thrombocytopenia. Platelet transfusion is indicated.
- The accompanied renal impairment may be due to either associated pneumonia or the co-existence of nephropathy.
- The only limitation of this case report is the contraindication of CTPA that can't be done due to the presence of renal impairment.
Conclusion and Recommendations
The presence of mimic COVID-19 pneumonia about 1 year before emerge of the COVID-19 pandemic is interesting and stupendous.
The association of Brugada syndrome, renal impairment, and thrombocytopenia in pneumonia are a constellation of serious risk factors.
Abbreviations
BrS: Brugada syndrome
COVID-19: Coronavirus disease 2019
CTPA: CT pulmonary angiogram
ECG: Electrocardiogram
ED: Emergency department
ICU: Intensive care unit
ITP: Immune thrombocytopenic purpura
O2: Oxygen
SGOT: Serum glutamic-oxaloacetic transaminase
SGPT: Serum glutamic-pyruvic transaminase
VR: Ventricular rate
Acknowledgment
I wish to thank the team of nurses in the critical care unit of Faraskour Central Hospital who make extra-ECG copies for helping me.
Conflicts of interest
There are no conflicts of interest.
References
- Ghoneim, A.H.A., Mohammad, M.A., Elghamrawy, M.A. et al. Platelet count as a predictor of outcome of hospitalized patients with community-acquired pneumonia at Zagazig University Hospitals, Egypt. Egypt J Bronchol 2020;14. DOI;https://doi.org/10.1186
Publisher | Google Scholor - Keni R, Alexander A, Nayak PG, Mudgal J and Nandakumar K (2020) COVID-19: Emergence, Spread, Possible Treatments, and Global Burden. Front. Public Health 8:216. DOI: 10.3389/fpubh.2020.00216
Publisher | Google Scholor - CDC. Pneumonia. Available online: https://www.cdc.gov/pneumonia/index.html (Accessed: March 9, 2020).
Publisher | Google Scholor - Elsayed YMH. An Electrocardiographic Triple Rhythm of Sinus Arrhythmia, Brugada Syndrome, And Early Repolarization with A Diverse Outcome. WW Med. 2019;1(9):303-306.DOI: 10.5455/ww.73149.
Publisher | Google Scholor - Magdi M, Rahil A. Severe immune thrombocytopenia complicated by intracerebral haemorrhage associated with coronavirus infection: a case report and literature review. EJCRIM. 2019;6. DOI:10.12890/2019_001155.
Publisher | Google Scholor - Taub JW, Warrier I, Holtkamp C, Beardsley DS, Lusher JM. Characterization of autoantibodies against the platelet glycoprotein antigens IIb/IIIa in childhood idiopathic thrombocytopenia purpura. Am J Hematol. 1995 Feb;48(2):104-7. DOI: 10.1002/ajh.2830480207. PMID: 7847322
Publisher | Google Scholor - Khan AI, Anwer F. Platelet Transfusion. [Updated 2022 May 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560632/
Publisher | Google Scholor