Research Article
Physical and Chemical Analysis of Soil Collected from Malakander Farm
1Department of Biochemistry, Abdul Wali Khan University Mardan, Pakistan.
2Department of Animal Nutrition the University of Agriculture Peshawar, Pakistan.
3Department of Chemistry and Biochemistry, the Agriculture University Peshawar, Pakistan.
4Department of Livestock and Dairy development Khyber Pukhtune Khwa, Pakistan.
*Corresponding Author: Sadiq Ullah, Department of Biochemistry, Abdul Wali Khan University Mardan, Pakistan.
Citation: Ullah S, Haris M, BiBi H, Alam T. (2024). Physical and Chemical Analysis of Soil Collected from Malakander Farm, Scientific Research and Reports, BioRes Scientia Publishers. 1(4):1-5. DOI: 10.59657/2996-8550.brs.24.031
Copyright: © 2024 Sadiq Ullah, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: August 30, 2024 | Accepted: September 16, 2024 | Published: September 19, 2024
Abstract
The physical and chemical properties of soil control the solubility and bioavailability of essential plant nutrients and thus establish a strong relationship between soil constituents and plant productivity. Thus, understanding these properties of soils as they affect agricultural productivity is extremely important. These studies cover some of the fundamental physiochemical properties (Ph, Ash, Moisture, Nitrogen content, Phosphorus content, OM) of soil constituents. The Ph of many Pakistani soil ranges from (7.5-8.5). The reason for high ph is the calcareous parent material, low rainfall, and low foret density. The micronutrient exception of the MO, are more soluble at low ph (5.0-6.5). Concentration of Fe+2 and Mn may reach toxic level in ph less than 5.0. Cations i.e., Ca2, Mg2 and Na exist in higher concentration in arid soil and are not very soluble in the neutral and alkaline ph range. The solubility of P is optimum over a narrow ph range (6.5-7.5). Ash determination of sample is part of the proximate analysis necessary for nutritional analysis. This ensures the safety of foods, making sure there are no toxic minerals present in soil. Moisture is a key variable in controlling the exchange of water and heat energy between the land surface and the atmosphere through evaporation and plant transpiration. As a result, soil moisture plays an important role in the development of weather patterns and the production of precipitation. Soil collected from Malakander farm Peshawar Pakistan. The result revealed that the moisture (6.1), ash (90.2), ph (6.7).
Keywords: plant nutrients; safety of foods; soil ranges; malakander farm
Introduction
Land use changes may result in the physical quality of the soil degrading, which might have detrimental consequences on the environment and agricultural output. Thus, it is crucial to keep an eye on the loss of biodiversity, water, and soil in watersheds. First, the physical-hydric characteristics of the soil must be described, as these cause transient alterations that might jeopardize the ecosystem's integrity [1]. Whereas the soils of River Meme ranged from slightly acidic to moderately acidic, all of the soils around River Wouri were somewhat acidic. They all displayed ΔpH variations that were consistently negative [pH (KCl)-pH (H2O)]. Although the mean ECEC was greater near the River Wouri, they had low ECEC and are likely dominated by kaolinitic minerals and sesquioxides, with limited retention capabilities [2]. A healthy soil is one that is continuously able to support biological production, improve the quality of the air and water environments, and uphold the health of people, animals, and plants while operating within ecological and land-use restrictions. Because it influences all other soil factors, the pH level of the soil is the most crucial factor. Consequently, pH is considered while analyzing any kind of soil. If the pH of a soil is less than 6, normal if it is between 6 and 8, and alkaline if it is more than 8.5, the soil is considered acidic [3]. In the 0-15 cm soil level, the pH varied from 4.71 to 6.01, and in the 15-30 cm soil depth, it ranged from 4.64 to 5.76. In the 0–15 cm depth range and the 15-30 cm depth range, the research area's soil organic carbon (SOC) varied from 3.9 to 17.0 g kg? 1 and 3.5 to 7.6 g kg-1. With the exception of CCL in the top soil, there was no statistically significant difference in Soil Organic Carbon across the land-use categories at the two depths. The land-use types' ionic exchange capacities varied from 2.32 to 3.18 cmol kg-1 at 0-15 cm depth and from 2.69 to 3.09 cmol kg-1 at 15-30 cm depth [4]. The organic carbon, phosphorus, potassium, and pH of the soil were studied physic-chemically using established procedures. The pH-Organic Carbon, pH-Phosphorus, and pH-Potassium Pearson association Coefficients show a somewhat negative association [5]. Soil response or pH can be used to examine the soil's acidity or alkalinity. Because it establishes capacity, the PH of the soil is a crucial feature. The soil's pH value indicates its acidity (<6>8.5). This [6] The investigated soils have different particle size distributions along the topo sequence. Across the profile, soils at the intermediate slope position had a comparatively greater clay concentration (25-85%), followed by soils at the upper (15-60%), toe (35-55%), and lower slope (25-30%) locations [7]. It eventually causes the pH of the soil system to drop, resulting in soil acidity. Soil acidification is the process by which soil pH gradually drops as a result of heavy rainfall and conventional farming practices [8]. Exchangeable Bases: 0.05N NH4OAc buffered at pH 7.0 was used to extract the soils (Ca, Mg, K, and Na). The extracts' exchangeable K and Na contents were measured using an EEL photometer. The titration technique was used to determine exchangeable Ca and Mg. Al3+, H+ combined, total exchangeable acidity 1 N KLC was used to extract and phenolphthalein was used as an indicator in the titration procedure using 0.05 N NAOH to determine the result. Exchangeable cation effective capacity value was determined by adding the exchangeable bases and total exchangeable acidity [9]. Heavy metal absorption and uptake into the food chain are influenced by the physicochemical characteristics of soils, particularly pH [10].
Materials and Methods
Soil Sampling
A soil sample should be composed of several sub-samples representing a seemingly uniform area or field with similar cropping and management history. Eight sub-samples are taken per hectare (ha) in a diagonal pattern for obtaining one composite sample. In this way three replicate of the compost soil was prepared.
Sampling Time
Soil samples can be taken any time that soil conditions permit, but sampling directly after fertilization or amendment application should be avoided. Samples were collected before sowing seed.
Sampling Tool
Soil samples for micronutrient analysis should be taken using a stainless-steel auger, or at least ungalvanized auger (because galvanized coating is zinc oxide). If you do not have sampling tools, use a spade as follows: Dig a V-shaped hole, 15 to 20 cm deep. Then take a fine thick slice from the smooth side.
Soil physiochemical properties
Soil pH is a crucial soil indicator, and is defined as the negative log of the hydrogen ion activity. Since pH is logarithmic, the H-ion concentration in solution increases ten times when its pH is lowered by one unit. The pH range normally found in soils varies from 3 to 9. Various categories of soil pH may be arbitrarily described as follows:
- Strongly acid (pH lessthan 5.0)
- Moderately to slightly acid (5.0-6.5).
- Neutral (6.5-7.5).
- Moderately alkaline (7.5-8.5).
- Strongly alkaline (>8.5).
Apparatus
PH meter with combined electrode, Reference electrode, saturated KCl, Measuring cylinder, Glass rod, Glass beaker, Interval timer, Wash bottle.
Reagents
- De-ionized water.
- PH 7.0 buffer solution.
- PH 4.0 buffer solution.
Procedure
- Weigh 50 g air-dry soil (lessthan2-mm) into a 100-mL glass beaker.
- Add 50 mL/dI water using a graduated cylinder or 50-mL volumetric flask.
- Mix well with a glass rod, and allow standing for 30 minutes.
- Stir suspensions every 10 minutes during this period.
- After 1 hour, stir the suspension.
- Calibrate the pH meter (see Box No. 3 for more details).
- Put the combined electrode in suspension (about 3-cm deep). Take the reading after 30 seconds with one decimal
- Remove the combined electrode from the suspension, and rinse thoroughly with DI water in a separate beaker, and carefully dry excess water with a tissue.
Soil Moisture Content
Soil moisture influences crop growth not only by affecting nutrient availability, but also nutrient transformations and soil biological behavior.
Apparatus: Electric oven with thermostat Desiccators
Procedure
- Weigh 10 g air-dry soil (<2>
- Dry in an oven, with the lid unfitted, at 105 °C overnight (normally for 24 hours).
- Next day, when the soil has dried, remove the container from the oven, using tongs; fit the lid, cool in a desiccator for at least 30 minutes and re-weigh.
Calculation:

Soil Organic Matter
(SOM) represents the remains of roots, plant material, and soil organisms in various stages of decomposition and synthesis, and is variable in composition. Soil Organic Carbon (SOC) ranges from being the dominant constituent of peat or muck soils in colder regions of the world to being virtually absent in some desert soils. Cultivated, temperate-region soils normally have often than 3-4 % SOM, while soils of semi-arid rained areas, such as in the WANA region, have normally less than 1.5 % SOM.
Apparatus: Magnetic stirrer and Teflon-coated magnetic stirring bar Glassware and pipettes for dispensing and preparing reagents Titration apparatus (burette)
Reagents
- Potassium Dichromate Solution (K2Cr2O7), 1N. Dry K2Cr2O7 in an oven at 105 °C for 2 hours. Cool in a desiccator (silica gel), and store in a tightly stopper bottle. Dissolve 49.04g K2Cr2O7 in DI water, and bring to 1-L volume.
- Sulfuric Acid (H2SO4) concentrated (98 %, sp. gr. 1.84)
- Orthophosphoric Acid (H3PO4), concentrated
- Ferrous Ammonium Sulfate Solution [(NH4) 2SO4.FeSO4.6H2O], 0.5M. Dissolve 196 g ferrous ammonium sulfate in DI water, and transfer to a 1-L flask, add 5 mL concentrated H2SO4, mix well, and bring to volume.
- Diphenylamine Indicator (C6H5)2NH. Dissolve 1 g diphenylamine indicator in 100 mL concentrated H2SO4.
Procedure
- Weigh 1 g air-dries soil (0.15 mm) into a 500-mL beaker.
- Add 10 mL 1 N potassium dichromate solution using a pipette, add 20 mL concentrated H2SO4 using a dispenser, and swirl the beaker to mix the suspension.
- Allow to stand for 30 minutes.
- Add about 200 mL DI water, then add 10 mL concentrated H3PO4 using a dispenser, and allow mixture to cool.
- Add 10-15 drops diphenylamine indicator, add a Teflon-coated magnetic stirring bar, and place the beaker on a magnetic stirrer.
- Titrate with 0.5 M ferrous ammonium sulfate solution, until the color changes from violet blue to green.
- Prepare two blanks, containing all reagents but no soil, and treat them in exactly the same way as the soil suspensions.
Calculation: M=10/V blank

Total organic carbon (%) = 1.334 x oxidizable carbon (%).
Organic matter (%) = 1.724 x total organic carbon (%).
Results
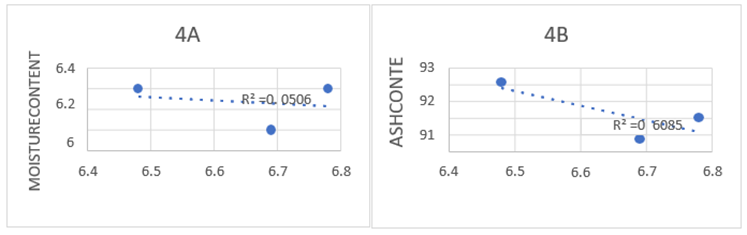
Analysis of various parameters of soil collected from Malakander farm area.
| Parameters | R1 | R2 | R3 | Average ± SD |
| pH | 6.78 | 6.69 | 6.48 | 6.68±0.173205 |
| MOISTURE | 6.2 | 5.8 | 6.2 | 6.0667±0.2309 |
| ASH | 90.0099 | 88.72 | 92.14 | 90.28997±1.72716 |
| PHOSPHOROS | 0.1 | 0.2 | 3.0 | 1.1±1.646208 |
| NITROGEN | 10.97 | 18.81 | 23.52 | 17.76667±6.33979 |
Figure: Relationship of ph with moisture content ash content of soil sample. Data are theme a value of three replicates of the sample collected from farm
Discussion
PH readings varied from 4.72 in Abraka to 6.52 in Sapele, indicating a range of acidity from extremely acidic to mildly acidic. However, the pH at the subsurface varied from 4.24 to 5.25, which is a comparable range from mildly acidic to extremely acidic. Except for Aghalokpe and Sapele, the pH of the soils decreased with depth. The pH readings of Abraka (4.72) (rain forest) at both locations were lower than the recommended range of 6-6.5 [9]. Crop productivity benefits from the exchange site of soils with a high Ca content, followed by Mg, K, and Na. With the exception of K in the middle, lower, and toe slope pedons, which displayed relative degradation, the exchangeable cations content of the soils rose with increasing soil depth. The increase was ascribed to exchangeable cation leaching [7]. Physical-chemical examination soil in various parts of Latur City came to the conclusion that several criteria may be used to assess soil quality. Because of the environmental conditions around them, the majority of the metrics are either fully above or lower than safe limits. One important factor that helps confirm the availability of plant nutrients is pH [5]. Because it influences all other soil factors, the pH level of the soil is the most crucial factor. Consequently, pH is considered while analyzing any kind of soil. If the pH of a soil is less than 6.0, it is referred to as acidic.
Conclusion
Deficiency of N reduces cell division, chlorophyll development and enzyme activity which result in stunted growth and yellow color. Excess of N leads to excess succulent growth of plants which causes susceptibility to insects, pests, disease. Fertile soils are characterized by their ability to support plant growth. This property depends on the presence of organic matter, which has a profound effect on all the soil functions related to its productivity. It has already been shown that soil organic matter serves not only as a reservoir of all these plant nutrients, but it also gives structure to the soil and provides energy for the microbial activity, which is essential for recycling of nutrients, thus maintaining the productivity of soil. In living plant, P play a vital role in several key physiological process, viz. photosynthesis, respiration, energy storage and transfer, cell division, cell enlargement, etc. High energy phosphate compounds are involved in almost every metabolic reaction in plants. In food material P is usually determined and express as phosphoric acid (P2O5). This may be done by colorimetric. Colorimetric determination is based upon the principal that contain element on a reaction with a suitable reagent develop color. The intensity of the color is the function of the concentration of the elements.
References
- Moratelli FA, Alves MAB, Borella DR, Kraeski A, Almeida FT, et al. (2023). Effects of Land Use on Soil Physical-Hydric Attributes in Two Watersheds in The Southern Amazon, Brazil. Soil Systems. 7(4):103.
Publisher | Google Scholor - Fomenky Norbert, Tening Aaron, George Bindeh, Mbene Kenneth, Asongwe Godswill, et al. (2018). Selected Physicochemical Properties and Quality of Soils Around Some Rivers of Cameroon. Journal Of Soil Science and Environmental Management. 9:68-80.
Publisher | Google Scholor - Vaibhav More, Pradip Dalavi, Kunal Rane. (2022). A Review on The Role of Soil Physico-Chemical Properties for Soil Quality Improvement and Sustainable Agriculture. The Pharma Innovation Journal. 11(11S):1598-1602.
Publisher | Google Scholor - Akinde Bamikole Peter, et al. (2020). Selected Physical And Chemical Properties Of Soil Under Different Agricultural Land-Use Types In Ile-Ife, Nigeria, Heliyon, 6(9):e05090.
Publisher | Google Scholor - Swami Swati, Jadhav Mahesh, Patil Abhijeet, Nandkumar Kalyani. (2019). Analysis of Physico-Chemical Properties of Soil from Latur City. Journal Of Emerging Technologies and Innovative Research. 6(5):3285-3290.
Publisher | Google Scholor - Karande Sucheta, Gamit Sheela, Prajapati Dhaval. (2020). Analysis Of Soil Samples for Its Physical and Chemical Parameters from Mehsana and Patan District, Int. Res. Journal of Science & Engineering, A9:86-94.
Publisher | Google Scholor - A. Ali, A. Esayas, S. Beyene, (2010). Characterizing Soils of Delbo Wegene Watershed, Wolaita Zone, Southern Ethiopia for Planning Appropriate Land Management, J. Soil Sci. Environ. Manag., 1(8):184-199.
Publisher | Google Scholor - Muche M, Kokeb A, Molla E. (2015). Assessing The Physicochemical Properties of Soil Under Different Land Use Types. J Environ Anal Toxicol. 5:309.
Publisher | Google Scholor - Umeri C, Onyemekonwu R, Moseri H. (2017). Analysis Of Physical and Chemical Properties of Some Selected Soils of Rain Forest Zones of Delta State, Nigeria. Agri Res & Tech: Open Access J. 5(4): 555668.
Publisher | Google Scholor - Undal V. S., (2021). Investigation On Physico-Chemical Parameters of Soil from Washim District of Maharashtra (India), Res. Rev. Int. J. Multidiscip., 6(1):23-29.
Publisher | Google Scholor