Case Report
Perioperative Management of an Esophageal Cancer Patient Undergoing Thoracoscopic Radical Esophageal Cancer Surgery with Hypophysitis Caused by Sintilimab
- Zhuo Wang, M.M. 1
- Ru-Ping Dai, M.D, Ph.D. 1
- Rong Yu, M.D, Ph.D. 1
- Zhen-Kun Xia, M.D, Ph.D. 2*
- Yan-Ling Zhang, M.D, Ph.D. 1
1Department of Anesthesiology, The Second Xiangya Hospital, Central South University, Changsha, China.
2Department of Thoracic Surgery, The Second Xiangya Hospital, Central South University, Changsha, China.
*Corresponding Author: Zhen-Kun Xia, Department of Thoracic Surgery, The Second Xiangya Hospital, Central South University, Changsha, China.
Citation: Wang Z, Ru-P. Dai, Yu R, Zhen-K. Xia, Yan-L. Zhang. (2025). Perioperative Management of an Esophageal Cancer Patient Undergoing Thoracoscopic Radical Esophageal Cancer Surgery with Hypophysitis Caused by Sintilimab. Clinical Case Reports and Studies, BioRes Scientia Publishers. 9(5):1-5. DOI: 10.59657/2837-2565.brs.25.235
Copyright: © 2025 Yan-Ling Zhang, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: February 10, 2025 | Accepted: February 24, 2025 | Published: March 03, 2025
Abstract
Background: Programmed cell death protein-1 (PD-1) inhibitor-induced hypophysitis is a rare adverse reaction that can cause adrenocortical insufficiency, leading to inadequate corticosteroid secretion and potential adrenal crises during surgery. This report discusses the perioperative management of a 59-year-old male with esophageal squamous cell carcinoma who developed hypophysitis and secondary adrenocortical insufficiency after treatment with the PD-1 inhibitor Sintilimab, and subsequently underwent thoracoscopic radical esophageal cancer surgery.
Case Presentation: After receiving two cycles of Sintilimab and neoadjuvant chemotherapy, the patient was diagnosed with diffuse pituitary inflammation and secondary adrenocortical insufficiency, likely caused by Sintilimab. Preoperative management included comprehensive endocrinological assessment and corticosteroid replacement therapy. The surgery was successfully performed with close intraoperative monitoring, appropriate corticosteroid administration, and timely glucocorticoid supplementation. The patient's postoperative recovery was uneventful.
Conclusion: PD-1 inhibitor-induced hypophysitis and subsequent adrenal insufficiency require vigilant perioperative management. Early detection, multidisciplinary team consultation, standardized corticosteroid replacement therapy, and regular follow-up are essential for ensuring patient safety and favorable surgical outcomes.
Keywords: programmed cell death protein-1; sintilimab; hypophysitis adrenocortical insufficiency
Introduction: Background
Neoadjuvant chemotherapy combined with immunotherapy followed by surgery is an effective treatment strategy for locally advanced esophageal squamous cell carcinoma [1, 2]. Immune checkpoint inhibitors (ICIs) have become vital in clinical anticancer therapy, including cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) inhibitors, programmed cell death protein-1 (PD-1) inhibitors, and programmed cell death ligand-1 (PD-L1) inhibitors [3]. Immunotherapy-induced hypophysitis (IH), particularly induced by PD-1 inhibitors, is a rare adverse event with an incidence of approximately 1% [4].
The diagnosis of IH is based primarily on clinical signs of pituitary hormone deficiency. Patients with adrenocortical insufficiency are at risk of inadequate corticosteroid secretion during stress, potentially leading to life-threatening adrenal crises during surgery. Hence, timely diagnosis, appropriate corticosteroid replacement therapy, and vigilant perioperative monitoring are crucial to ensure patient safety.
In this report, we present a case of perioperative management for a patient with esophageal cancer who developed hypophysitis and secondary adrenocortical insufficiency after treatment with the PD-1 inhibitor Sintilimab, undergoing thoracoscopic radical esophageal cancer surgery. This case underscores the importance of timely diagnosis, glucocorticoid replacement therapy, and meticulous intraoperative monitoring in managing such patients.
Case Presentation
A 59-year-old Chinese male was diagnosed with esophageal squamous cell carcinoma (CT3N1M0) at a local hospital on August 31, 2023. He received two cycles of chemotherapy combined with Sintilimab 200 mg (d1), aztreximab 370 mg (d1), and cisplatin 35 mg (d1, d2, d3) on September 7, 2023, and October 10, 2023. Before immunotherapy, his hepatic and renal functions, endocrine function, and electrolytes were normal, and his condition remained stable.
On December 3, 2023, the patient presented with poor mental health, fatigue, loss of appetite, nausea, and vomiting. Laboratory tests revealed adrenocortical insufficiency, with corticosteroid levels below 13.8 nmol/L at 00:00, 08:00, and 16:00, and a downward trend in Adrenocorticotropic hormone (ACTH) levels during immunotherapy. Thyroid function was normal, and gonadal function was not tested due to his age. Other tests, including electrocardiogram, echocardiogram, pulmonary function, and head magnetic resonance imaging (MRI) scan, were normal. He was prescribed hydrocortisone acetate tablets (20 mg at 8:00 AM and 10 mg at 16:00 PM) for replacement therapy. Two weeks later, his symptoms improved, and he was readmitted for surgery.
The patient was scheduled for thoracoscopic radical esophageal cancer surgery. The perioperative management was discussed by a multidisciplinary team (MDT) of anesthesiologists, endocrinologists, and surgeons. They confirmed the diagnosis of hypocortisolism due to diffuse pituitary inflammation caused by PD-1 immunotherapy and planned corticosteroid substitution therapy for the perioperative period, including preoperative, intraoperative, and postoperative phases (Table 1). Continuous monitoring of direct arterial pressure, glucose, electrolytes, and temperature was necessary to prevent and detect early signs of cortisol crisis.
Table 1: Hormone level for the patient
| Date | Hormone | ||
| ACTH (0-46 ng/L) | Cortisol (85.3-618nmol/L) | TSH (0.30-4.50 pmol/L) | |
| 2023.09.06 | 26.2 | 651.1 | / |
| 2023.10.10 | 14.8 | 344.8 | / |
| 2023.12.04 | 9.2 | 14.9 | 1.68 |
| 2023.12.05 | / | less than 13.8 (0:00am;8:00am;16:00pm) | / |
| 2023.12.08 | 6.6 | less than 13.8 (0:00am;8:00am;16:00pm) | / |
| 2023.12.21 | / | 592.2;862.2;974 (0:00am; 8:00am; 16:00pm) | / |
| 2024.02.26 | <5> | 29.9 | 2.89 |
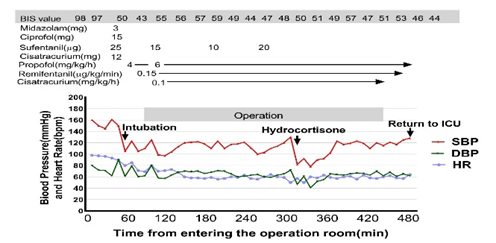
The surgery was performed on December 25, 2023. Before the operation, the patient received 200 mg of hydrocortisone intravenously at 6:00 AM. After anesthesia induction, a double-lumen tube was placed for one-lung ventilation, and the patient was monitored with electrocardiogram, pulse oximetry, invasive blood pressure, bispectral index, end-tidal carbon dioxide, and blood gases. During the surgery, blood pressure fluctuated between 129/79 mmHg and 158/73 mmHg until 13:10 PM, when it dropped to between 82/47 mmHg and 90/52 mmHg (Figure 1). Despite repeated doses of norepinephrine, hypotension persisted. This was attributed to corticosteroid deficiency, and an additional 100 mg of hydrocortisone was administered, normalizing blood pressure within 30 minutes. Arterial blood gas analysis showed no hypoglycemia or hyponatremia during surgery.
The surgery lasted six hours, with an intraoperative infusion volume of 2600 ml, blood loss of 800 ml, and urine output of 1000 ml. Postoperatively, the patient was transferred to the Thoracic Intensive Care Unit, where hydrocortisone was administered as scheduled. He was discharged on January 9, 2024, with improved mental status and prescribed hydrocortisone acetate tablets (20 mg at 8:00 AM and 10 mg at 16:00 PM) for follow-up therapy. Hormone levels normalized post-surgery (Table 1). The patient returned to the hospital on February 26, 2024, for postoperative immunotherapy with a normal mental status.
Discussion
Immunotherapy, particularly immune checkpoint inhibitors (ICIs), is increasingly used before surgical resection of cancers. ICIs, including CTLA-4 and PD-1 inhibitors, play a key role in maintaining immunological tolerance and preventing autoimmune disorders [3]. However, by activating T cells against cancer, ICIs can also trigger immune-related adverse events (irAEs) affecting various organs and systems, such as the skin, lungs, and endocrine system [5-7]. Endocrine dysfunctions are among the most common irAEs, with immunotherapy-induced hypophysitis (IH) being a rare but significant adverse event. The incidence of IH induced by PD-1 inhibitors is approximately 1% [4].
Early diagnosis and aggressive treatment of IH are crucial for the prognosis of patients undergoing surgery, as this condition can lead to life-threatening perioperative adrenocortical crises. IH can impact thyroid, adrenal, and gonadal functions, either simultaneously or individually [8]. A retrospective analysis indicated that the onset of IH induced by PD-1/PD-L1 antibodies can vary from a few weeks to a year [9]. In this case, the patient, who had no prior endocrine dysfunction, developed symptoms of dysphoria, malaise, loss of appetite, nausea, vomiting, and decreased cortisol levels 53 days after starting Sintilimab treatment. Laboratory tests showed persistent cortisol levels below 13.8 nmol/L, while ACTH levels were normal. Upon readmission for postoperative immunotherapy, the patient's ACTH levels were below 5 ng/L. Similar delayed ACTH changes relative to cortisol levels were reported in four patients with isolated secondary adrenocortical insufficiency induced by PD-1 blockade [10].
The diagnosis of IH relies on clinical manifestations of pituitary hormone deficiency, including malaise, anorexia, nausea, vomiting, unexplained fever, hypotension, hypoglycemia, and hyponatremia [11]. Despite normal cranial MRI findings, the patient's clinical presentation and laboratory tests confirmed IH with diffuse pituitary inflammation and secondary adrenocortical insufficiency. Treatment mainly involves hormone replacement therapy, often requiring lifelong corticosteroid supplementation.
Surgical stress, anesthesia depth, and individual susceptibility contribute to the intraoperative stress response [12]. Patients with adrenal insufficiency lack the appropriate cortisol response to surgical stress, leading to hypotension due to loss of vasomotor tone [12]. It is critical to design a perioperative steroid supplementation plan and closely monitor blood pressure, glucose, electrolytes, and temperature [13]. In this case, the MDT protocol included administering 200 mg of hydrocortisone intravenously before surgery. However, the patient's blood pressure dropped during surgery, unresponsive to vasoactive drugs (Figure 1). After excluding surgical and anesthetic factors, the intraoperative hypotension was attributed to corticosteroid deficiency. Supplementing with 100 mg of hydrocortisone corrected the hypotension within 30 minutes.
Figure 1: Temporal Changes of Perioperative Hemodynamic Parameters. SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate
IH is a rare adverse reaction that can be easily overlooked by surgeons and anesthesiologists. Prompt diagnosis, glucocorticoid replacement therapy, and close intraoperative monitoring are essential to prevent perioperative adrenocortical crises and ensure favorable patient outcomes. Anesthesiologists play a crucial role in the perioperative management of patients with IH. In this case, a perioperative MDT led by an anesthesiologist ensured patient safety, highlighting the importance of interdisciplinary collaboration in perioperative care.
Conclusion
Immunotherapy-induced hypophysitis (IH) is a rare but serious condition that requires prompt diagnosis and glucocorticoid replacement therapy. Close intraoperative monitoring is essential to prevent life-threatening adrenocortical crises during surgery. This case highlights the importance of a multidisciplinary team (MDT) approach, involving anesthesiologists, endocrinologists, and surgeons, to ensure patient safety and achieve successful surgical outcomes. Effective management of IH requires careful planning and collaboration among healthcare professionals.
List of abbreviations
PD-1: Programmed cell death protein-1;
ICIs: Immune checkpoint inhibitors;
CTLA-4: Cytotoxic T lymphocyte-associated antigen-4;
PD-L1: Programmed cell death ligand-1;
IH: Immunotherapy-induced hypophysitis;
ACTH: Adrenocorticotropic hormone;
MRI: Magnetic Resonance Imaging;
MDT: Multidisciplinary Team;
IrAEs: Immune-related adverse events;
ICU: Intensive Care Unit.
Declarations
Ethics approval and consent to participate
This case report was approved by the Ethics Committee of the Second Xiangya Hospital, Central south University (LYEC2024-K0036). After explaining the study protocol of this case report to the patient, we have obtained written informed consent for study participation from the patient.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and the accompanying in ages.
Availability of data and materials
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Funding
Not applicable.
Authors' contributions
Zhuo Wang wrote the manuscript. Ru-Ping Dai and Rong Yu managed the anesthesia in this patient and collected the perioperative data. Yan-Ling Zhang and Zhen-Kun Xia revised the manuscript. All authors have read and approved the final manuscript.
Acknowledgements
Not applicable.
References
- Yu, Y. K., Meng, F. Y., Wei, X. F., Chen, X. K., Li, H. M., Liu, Q., et al. (2024). Neoadjuvant chemotherapy combined with immunotherapy versus neoadjuvant chemoradiotherapy in patients with locally advanced esophageal squamous cell carcinoma. Journal of Thoracic and Cardiovascular Surgery.
Publisher | Google Scholor - Hong, Z. N., Zhang, Z., Chen, Z., Weng, K., Peng, K., Lin, J., et al. (2022). Safety and feasibility of esophagectomy following combined neoadjuvant immunotherapy and chemotherapy for locally advanced esophageal cancer: A propensity score matching. Esophagus, 19(2):224-232.
Publisher | Google Scholor - Naidoo, J., Page, D. B., & Wolchok, J. D. (2014). Immune checkpoint blockade. Hematology/Oncology Clinics of North America, 28(3)585-600.
Publisher | Google Scholor - de Filette, J., Andreescu, C. E., Cools, F., Bravenboer, B., & Velkeniers, B. (2019). A systematic review and meta-analysis of endocrine-related adverse events associated with immune checkpoint inhibitors. Hormone and Metabolic Research, 51(3):145-156.
Publisher | Google Scholor - Barroso-Sousa, R., Barry, W. T., Garrido-Castro, A. C., Hodi, F. S., Min, L., Krop, I. E., et al. (2018). Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: A systematic review and meta-analysis. JAMA Oncology, 4(2):173-182.
Publisher | Google Scholor - Johnson, D. B., Nebhan, C. A., Moslehi, J. J., & Balko, J. M. (2022). Immune-checkpoint inhibitors: Long-term implications of toxicity. Nature Reviews Clinical Oncology, 19(4):254-267.
Publisher | Google Scholor - Lemiale, V., Meert, A. P., Vincent, F., Darmon, M., Bauer, P. R., Van de Louw, A., et al. (2019). Severe toxicity from checkpoint protein inhibitors: What intensive care physicians need to know? Annals of Intensive Care, 9(1):25.
Publisher | Google Scholor - Byun, D. J., Wolchok, J. D., Rosenberg, L. M., & Girotra, M. (2017). Cancer immunotherapy - immune checkpoint blockade and associated endocrinopathies. Nature Reviews Endocrinology, 13(4):195-207.
Publisher | Google Scholor - Di Dalmazi, G., Ippolito, S., Lupi, I., & Caturegli, P. (2019). Hypophysitis induced by immune checkpoint inhibitors: A 10-year assessment. Expert Review of Endocrinology & Metabolism, 14(6):381-398.
Publisher | Google Scholor - Yamauchi, I., Taura, D., Hakata, T., Fujita, H., Okamoto, K., Ueda, Y., et al. (2021). Clinical features and thyroid dysfunction in adverse events involving the pituitary gland during PD-1 blockade therapy. Clinical Endocrinology (Oxford), 94(2):258-268.
Publisher | Google Scholor - Joshi, M. N., Whitelaw, B. C., Palomar, M. T., Wu, Y., & Carroll, P. V. (2016). Immune checkpoint inhibitor-related hypophysitis and endocrine dysfunction: Clinical review. Clinical Endocrinology (Oxford), 85(3):331-339.
Publisher | Google Scholor - Woodcock, T., Barker, P., Daniel, S., Fletcher, S., Wass, J. A. H., Tomlinson, J. W., et al. (2020). Guidelines for the management of glucocorticoids during the peri-operative period for patients with adrenal insufficiency: Guidelines from the Association of Anaesthetists, the Royal College of Physicians and the Society for Endocrinology UK. Anaesthesia, 75(5):654-663.
Publisher | Google Scholor - Seo, K. H. (2021). Perioperative glucocorticoid management based on current evidence. Anesthesia & Pain Medicine (Seoul), 16(1):8-15.
Publisher | Google Scholor