Review Article
Pembrolizumab-Induced Renal Adverse Events: A Comprehensive Overview of Acute Interstitial Nephritis and Beyond
Norton Community Hospital, Norton, Virginia, United States.
*Corresponding Author: Supriya Peshin, Norton Community Hospital, Norton, Virginia, United States.
Citation: Peshin S, Patel P, Sonar N, Sadiq Z, Kaur G, et al. (2024). Pembrolizumab-Induced Renal Adverse Events: A Comprehensive Overview of Acute Interstitial Nephritis and Beyond, Journal of Hematology Research and Blood Disorders, BioRes Scientia Publishers. 1(1):1-7. DOI: 10.59657/jhrbd.brs.24.004
Copyright: © 2024 Supriya Peshin, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: August 26, 2024 | Accepted: September 30, 2024 | Published: October 07, 2024
Abstract
Pembrolizumab, an immune checkpoint inhibitor targeting PD-1, has revolutionized cancer therapy by enhancing anti-tumor immune responses. However, it is associated with immune-related adverse events (irAEs), including acute interstitial nephritis (AIN) and other renal toxicities. This review discusses the pathophysiology, clinical manifestations, diagnosis, and management of pembrolizumab-induced AIN and other renal side effects, emphasizing the importance of early detection and intervention.
Keywords: pembrolizumab; immune checkpoint inhibitors; acute interstitial nephritis
Introduction
The advent of immune checkpoint inhibitors (ICIs) has significantly transformed cancer therapy, offering new hope for patients with advanced cancers. Pembrolizumab (Keytruda) is an immune checkpoint inhibitor that targets programmed cell death protein 1 (PD-1) and has become a cornerstone in the treatment of numerous malignancies, including melanoma, non-small cell lung cancer, renal cell carcinoma, and Hodgkin lymphoma. By blocking the PD-1 pathway, pembrolizumab reinvigorates T-cell activity, allowing the immune system to recognize and destroy cancer cells more effectively. While this mechanism has yielded remarkable therapeutic outcomes, it also predisposes patients to immune-related adverse events (irAEs), which can affect various organs, including the kidneys.
Pembrolizumab-induced kidney damage, although less frequent than other irAEs, presents significant clinical challenges. Acute tubulointerstitial nephritis (ATIN) is the most common renal manifestation, although other forms of glomerular involvement have been reported. The pathophysiology of these kidney injuries involves complex immune-mediated processes, which remain under investigation. Understanding the incidence, risk factors, and management strategies for pembrolizumab-induced renal damage is crucial for oncologists and nephrologists to mitigate adverse outcomes and optimize cancer treatment (Cortazar et al., 2016; Izzedine et al., 2014).
Pathophysiology
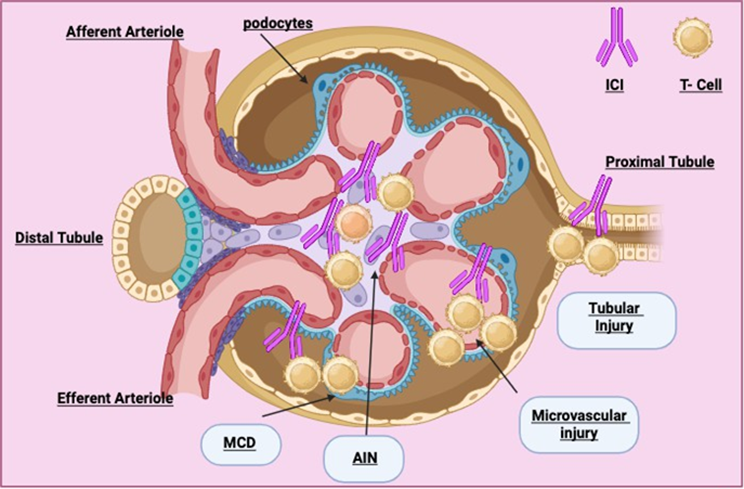
Pembrolizumab-induced acute interstitial nephritis (AIN) is a manifestation of the immune-mediated side effects associated with immune checkpoint inhibitors. The underlying pathophysiology is complex and involves several key mechanisms:
Immune Checkpoint Inhibition and T-Cell Activation: Pembrolizumab is an immune checkpoint inhibitor that targets programmed cell death protein 1 (PD-1). PD-1 is a receptor on the surface of T cells that normally functions to downregulate immune responses and maintain self-tolerance by inhibiting excessive T-cell activity. By blocking PD-1, pembrolizumab reduces these inhibitory signals, enhancing T-cell activation and proliferation. While beneficial for attacking cancer cells, this heightened immune response can also result in the loss of self-tolerance and the development of autoimmune conditions, including AIN (Shirali & Perazella, 2016; Belliere et al., 2016).
Autoimmune Reaction and Self-Tolerance Loss: The disruption of the PD-1 pathway leads to a breakdown in the mechanisms that normally prevent the immune system from attacking the body’s tissues. This loss of self-tolerance can cause T cells to target normal, healthy cells, including those in the kidneys. In the case of AIN, this autoimmune reaction is directed against renal tubular cells and interstitial tissues. The activation of autoreactive T cells, particularly CD4+ lymphocytes, contributes to the development of local inflammation within the renal interstitium. This inflammation is characterized by the infiltration of these lymphocytes into the kidney interstitium, where they release cytokines and other inflammatory mediators that further exacerbate tissue damage (Belliere et al., 2016).
Infiltration and Inflammation in Renal Tissue: In the kidneys, immune-mediated inflammation manifests as an infiltration of CD4+ T lymphocytes into the renal interstitium. These autoreactive T cells release pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6), contributing to the inflammatory cascade. These inflammatory cells and cytokines lead to localized tissue damage, including the destruction of renal tubular cells and disruption of normal renal function. This inflammation and subsequent tissue damage are hallmarks of AIN and can result in a reduction in the kidney’s ability to filter waste products from the blood, leading to acute kidney injury (Shirali & Perazella, 2016).
Histopathological Features: Histologically, AIN is characterized by the presence of lymphocytic infiltration in the renal interstitium, which can also include eosinophilic infiltration in some cases. The infiltrating lymphocytes disrupt the normal architecture of the renal interstitium and contribute to the clinical manifestations of AIN, such as elevated serum creatinine and reduced renal function. These histopathological changes underscore the immune-mediated nature of the condition and highlight the role of pembrolizumab-induced immune dysregulation in driving the development of renal toxicity (Belliere et al., 2016).
In summary, the pathophysiology of pembrolizumab-induced AIN involves the inhibition of PD-1, leading to increased T-cell activation and a subsequent loss of self-tolerance (table 1). This results in the infiltration of autoreactive CD4+ lymphocytes into the renal interstitium, where they induce local inflammation and tissue damage. Understanding these immune-mediated mechanisms is crucial for developing effective management strategies for patients experiencing AIN as a side effect of pembrolizumab therapy.
Figure 1: Pembrolizumab induced nephrotoxicity.
Table 1: Pathophysiology of Pembrolizumab-Induced Acute Interstitial Nephritis (AIN).
| Mechanism | Description |
| Immune Checkpoint Inhibition and T-cell activation | Pembrolizumab blocks PD-1, reducing inhibitory signals, and leading to enhanced T-cell activation and proliferation. This can cause loss of self-tolerance and autoimmune conditions. |
| Autoimmune Reaction and Self-Tolerance Loss | The disruption of the PD-1 pathway causes T cells to target healthy renal cells, leading to local inflammation in the renal interstitium. |
| Infiltration and Inflammation in Renal Tissue | CD4+ T lymphocytes infiltrate the renal interstitium, releasing cytokines like TNF-alpha and IL-6, causing tissue damage and acute kidney injury (AKI). |
| Histopathological Features | Histology shows lymphocytic and sometimes eosinophilic infiltration in the renal interstitium, disrupting normal architecture and leading to renal dysfunction. |
Clinical Manifestations
Pembrolizumab-induced renal toxicity primarily manifests as acute kidney injury (AKI), which can present with a variety of clinical and laboratory findings. The key features include:
Elevated Serum Creatinine: One of the hallmark signs of pembrolizumab-induced renal toxicity is a sudden increase in serum creatinine levels. This elevation reflects impaired renal function and is often one of the earliest indicators of acute kidney injury. Elevated serum creatinine results from a decrease in the kidney's ability to filter waste products from the blood effectively. Monitoring serum creatinine is crucial for detecting renal impairment, especially in patients undergoing pembrolizumab therapy, as it helps to identify early changes in kidney function that may necessitate intervention (Johnson et al., 2023).
Tubulointerstitial Nephritis (AIN): Renal biopsy is often required to confirm the diagnosis of pembrolizumab-induced renal toxicity. The biopsy typically reveals features of acute tubulointerstitial nephritis (AIN), characterized by lymphocytic infiltration and damage to the renal tubules. AIN is an immune-mediated condition in which the interstitium of the kidneys becomes inflamed, leading to renal dysfunction. Histopathological examination may show not only lymphocytic infiltration but also damage to the renal tubular epithelium. These findings are indicative of the immune-mediated injury associated with pembrolizumab therapy and are essential for distinguishing AIN from other causes of kidney injury (Belliere et al., 2016; Cortazar et al., 2024).
Proteinuria and Hematuria: In addition to elevated serum creatinine, patients with pembrolizumab-induced renal toxicity may exhibit proteinuria and hematuria. Proteinuria refers to the presence of abnormal amounts of protein in the urine, while hematuria indicates the presence of red blood cells. These findings are typically mild to moderate but can be indicative of underlying renal damage. Proteinuria and hematuria are important diagnostic markers that, when present, further support the diagnosis of renal impairment and can help guide the management and follow-up of affected patients (Shirali & Perazella, 2016).
Electrolyte Imbalances: Pembrolizumab-induced renal toxicity can also lead to disturbances in electrolyte balance, including hyponatremia and hyperkalemia. Hyponatremia, or low sodium levels, can occur due to impaired kidney function and can lead to various clinical symptoms, including confusion and weakness. Hyperkalemia, or elevated potassium levels, is another potential complication that can result from decreased renal excretion of potassium. Both of these electrolyte imbalances can exacerbate the clinical condition and complicate the management of renal toxicity. Addressing these imbalances through appropriate medical interventions is essential to prevent severe complications and ensure optimal patient outcomes (Izzedine et al., 2014; Cortazar et al., 2024).
In summary, pembrolizumab-induced renal toxicity typically presents as acute kidney injury with elevated serum creatinine, tubulointerstitial nephritis revealed by renal biopsy, proteinuria, hematuria, and potential electrolyte imbalances. Recognizing these clinical and laboratory features is crucial for the timely diagnosis and effective management of renal complications associated with pembrolizumab therapy.
Incidence and Risk Factors
The incidence of renal irAEs in patients receiving pembrolizumab varies, with studies reporting rates between 2% and 5%. AIN is the most common renal manifestation, accounting for 70%-90% of cases. Risk factors include pre-existing renal impairment, concurrent use of nephrotoxic medications (e.g., NSAIDs, PPIs), combination immunotherapy, and prolonged treatment duration (Cortazar et al., 2016; Kitchlu et al., 2023).
Diagnosis
Timely diagnosis of pembrolizumab-induced acute interstitial nephritis (AIN) is essential for effective management and minimizing renal damage. The diagnostic process should be comprehensive, incorporating a range of approaches to accurately identify and confirm the condition.
Medical History and Physical Examination: A thorough assessment of the patient’s medical history is fundamental in diagnosing AIN. Clinicians should inquire about the recent use of pembrolizumab and other medications, particularly those with known nephrotoxic potential. Additionally, the physical examination should focus on clinical signs of renal dysfunction, such as edema, hypertension, and symptoms of volume overload, which can provide early clues to the presence of kidney injury (Belliere et al., 2016).
Renal Function Tests: Monitoring renal function is crucial for diagnosing and managing AIN. Serum creatinine and blood urea nitrogen (BUN) levels are key indicators of kidney function and should be measured regularly. Elevated levels of these biomarkers can suggest renal impairment, prompting further investigation into the underlying cause of the dysfunction. Trends in these values over time can also provide insights into the progression of kidney damage and the effectiveness of therapeutic interventions (Cortazar et al., 2024).
Urinalysis: Urinalysis is an important diagnostic tool for evaluating renal involvement. The presence of proteinuria, hematuria, and pyuria can indicate glomerular or tubular damage. Proteinuria reflects damage to the glomerular filtration barrier, while hematuria and pyuria may suggest inflammatory processes in the renal interstitium or other parts of the urinary tract. These findings can help differentiate AIN from other types of renal injury (Izzedine et al., 2014).
Serologic Tests: To rule out other potential causes of renal disease, serologic tests are necessary. Tests for antinuclear antibodies (ANA) and antineutrophil cytoplasmic antibodies (ANCA) are commonly employed to exclude autoimmune conditions and vasculitis, which can present with similar renal symptoms. These tests help narrow down the differential diagnosis and confirm that the renal damage is attributable to pembrolizumab-induced AIN rather than an alternative etiology (Shirali & Perazella, 2016).
Imaging Studies: Renal imaging, particularly renal ultrasound, is used to exclude obstructive causes of kidney injury. Imaging studies can identify structural abnormalities, such as hydronephrosis or kidney stones, which might contribute to renal impairment. While imaging alone cannot diagnose AIN, it provides valuable information that helps to rule out other potential causes of renal dysfunction (Johnson et al., 2023).
Renal Biopsy: Renal biopsy remains the definitive diagnostic tool for confirming AIN. Histopathological examination of renal tissue typically reveals characteristic features such as lymphocytic infiltration in the interstitium, and in some cases, eosinophilic infiltration. These findings are crucial for distinguishing AIN from other forms of renal injury and guiding appropriate treatment strategies (Cortazar et al., 2024). A renal biopsy is particularly important when clinical and laboratory findings suggest AIN but are not definitive, ensuring that the diagnosis is accurate and guiding the management plan effectively.
In summary, the diagnosis of pembrolizumab-induced AIN involves a combination of assessing medical history, conducting physical examinations, performing renal function tests, urinalysis, serologic tests, imaging studies, and ultimately, renal biopsy. Each diagnostic approach contributes to a comprehensive evaluation, ensuring accurate identification of AIN and facilitating timely and effective management (Table 2).
Table 2: Diagnostic Approaches for Pembrolizumab-Induced AIN.
| Diagnostic Tool | Description |
| Medical History and Physical Examination | Assess recent use of pembrolizumab, other medications, and clinical signs of renal dysfunction. |
| Renal Function Tests | Regular measurement of serum creatinine and BUN levels to monitor renal function. |
| Urinalysis | Detects proteinuria, hematuria, and pyuria, indicating renal damage. |
| Serologic Tests | Excludes other causes of renal disease by testing for antinuclear antibodies (ANA) and antineutrophil cytoplasmic antibodies (ANCA). |
| Imaging Studies | Renal ultrasound to rule out obstructive causes of kidney injury. |
| Renal Biopsy | A definitive diagnosis was made through histopathological examination, which showed lymphocytic infiltration in the renal interstitium. |
Management Strategies
Management of pembrolizumab-induced acute interstitial nephritis (AIN) requires a comprehensive, multidisciplinary approach to optimize patient outcomes and mitigate renal damage. The key components of management include corticosteroid therapy, temporary discontinuation of pembrolizumab, supportive care, and, if necessary, alternative immunosuppression.
Corticosteroid Therapy: The cornerstone of treatment for pembrolizumab-induced AIN is corticosteroid therapy. Prednisone or methylprednisolone is commonly used to reduce inflammation and facilitate renal recovery. The recommended starting dose of prednisone is typically 0.5-1 mg/kg/day. This dose is usually tapered over 4-6 weeks based on the patient's clinical response and improvement in renal function (Shirali & Perazella, 2016; Sharma et al., 2023). Corticosteroids work by dampening the excessive immune response that contributes to renal inflammation and injury, thus aiding in the restoration of normal kidney function.
Temporary Discontinuation of Pembrolizumab: In cases of moderate to severe acute kidney injury (AKI), it is crucial to temporarily discontinue Pembrolizumab. This step is recommended to allow the kidneys to recover and to prevent further exacerbation of renal damage. During this period, close monitoring of renal function is essential. Reintroduction of pembrolizumab may be considered if renal function stabilizes and if the patient does not experience other severe immune-related adverse events (irAEs) (Johnson et al., 2023). The decision to rechallenge with pembrolizumab should be made cautiously, weighing the potential benefits of continued cancer treatment against the risks of recurrent renal toxicity.
Supportive Care: Supportive care plays a vital role in managing pembrolizumab-induced AIN. Ensuring adequate hydration helps to maintain proper kidney function and prevent complications such as dehydration. Correction of electrolyte imbalances, such as hyperkalemia and hyponatremia, is necessary to stabilize the patient’s condition. Additionally, it is important to avoid the use of nephrotoxic drugs, including non-steroidal anti-inflammatory drugs (NSAIDs) and certain antibiotics, which can exacerbate renal injury (Izzedine et al., 2014). Supportive care measures help to alleviate symptoms and support renal recovery, complementing the effects of corticosteroid therapy.
Alternative Immunosuppression: For patients who do not respond adequately to corticosteroids or who exhibit steroid-refractory AIN, additional immunosuppressive therapies may be required. Agents such as mycophenolate mofetil or azathioprine can be considered as second-line treatments. These medications work by further suppressing the immune system to control inflammation and prevent ongoing kidney damage (Cortazar et al., 2024). The choice of alternative immunosuppressive agents depends on the patient's overall health, the severity of the renal impairment, and the response to initial corticosteroid treatment.
In summary, the management of pembrolizumab-induced AIN involves a combination of corticosteroid therapy to address inflammation, temporary discontinuation of the drug to prevent further renal damage, supportive care to maintain kidney function, and alternative immunosuppressive treatments for refractory cases. A tailored approach, guided by the severity of the renal impairment and the patient’s response to initial treatments, is essential for optimizing outcomes and minimizing the long-term impact of renal toxicity.
Monitoring and Prevention
Regular Monitoring and Early Intervention
Baseline Assessment: Conducting a thorough baseline assessment of renal function is crucial before initiating treatment with pembrolizumab. This initial evaluation includes measuring serum creatinine and urinalysis to establish a patient’s pre-treatment renal status. Establishing baseline renal function helps in identifying deviations from normal values that may occur during treatment, thereby facilitating timely intervention should renal complications arise. This proactive approach allows for better management of potential adverse renal events and helps in distinguishing pembrolizumab-induced nephrotoxicity from pre-existing renal issues (Keytruda Prescribing Information).
Periodic Monitoring: Regular monitoring of renal function is essential for early detection and management of pembrolizumab-induced nephrotoxicity. Serum creatinine levels and urinalysis should be conducted at regular intervals, especially during the first 3-6 months of therapy when the risk of acute interstitial nephritis (AIN) is highest. Frequent monitoring helps in promptly identifying any increase in creatinine levels or changes in urine composition, which may signal the onset of renal impairment. By catching these changes early, clinicians can adjust treatment strategies and initiate appropriate interventions to prevent progression to more severe renal damage (Kitchlu et al., 2023).
Patient Education: Educating patients about the potential signs and symptoms of renal dysfunction is a key component of managing pembrolizumab-induced nephrotoxicity. Patients should be informed about symptoms such as decreased urine output, swelling, fatigue, and changes in urine color. Encouraging patients to promptly report any such symptoms can lead to earlier diagnosis and treatment, reducing the risk of severe renal complications. Effective patient education empowers individuals to be proactive in their healthcare, which is critical for managing the adverse effects of immunotherapy (Keytruda Prescribing Information).
Emerging Therapies and Future Directions
Biomarkers for Early Detection: Ongoing research is focused on identifying specific biomarkers that can enable the early detection of pembrolizumab-induced nephrotoxicity. Biomarkers could provide valuable insights into the onset of renal injury before clinical symptoms become apparent. By integrating biomarkers into routine monitoring, clinicians could potentially intervene earlier and implement preventive strategies to mitigate renal damage.
Advances in biomarker research hold promise for improving the precision and effectiveness of monitoring protocols (Cortazar et al., 2024).
Personalized Medicine: The development of personalized medicine approaches may enhance the management of pembrolizumab-induced nephrotoxicity by identifying patients at higher risk for renal complications. Genetic profiling and other individualized assessments could help tailor therapy based on a patient’s unique genetic and molecular characteristics. This approach may improve patient outcomes by allowing for more precise adjustments in treatment plans and preventive measures, ultimately reducing the incidence of severe renal adverse events (Kitchlu et al., 2023).
Novel Immunomodulatory Therapies: Research into novel immunomodulatory therapies aims to find agents that can modulate immune responses without compromising the anti-tumor efficacy of treatments like pembrolizumab. New agents and therapeutic strategies that target specific immune pathways involved in renal toxicity could potentially reduce the risk of nephrotoxicity while maintaining or enhancing anti-cancer activity. These innovations represent an exciting area of research and may provide new options for managing pembrolizumab-induced nephrotoxicity in the future (Cortazar et al., 2024).
In summary, regular monitoring, baseline assessment, and patient education are critical for preventing and managing pembrolizumab-induced renal complications. As research continues to evolve, emerging therapies and advancements in personalized medicine offer hope for improving the management of nephrotoxicity associated with immune checkpoint inhibitors, potentially leading to safer and more effective cancer treatments.
Conclusion
The advent of immune checkpoint inhibitors (ICIs) has significantly transformed cancer therapy, offering new hope for patients with advanced cancers. Pembrolizumab (Keytruda) is an immune checkpoint inhibitor that targets programmed cell death protein 1 (PD-1) and has become a cornerstone in the treatment of numerous malignancies, including melanoma, non-small cell lung cancer, renal cell carcinoma, and Hodgkin lymphoma. By blocking the PD-1 pathway, pembrolizumab reinvigorates T-cell activity, allowing the immune system to recognize and destroy cancer cells more effectively. While this mechanism has yielded remarkable therapeutic outcomes, it also predisposes patients to immune-related adverse events (irAEs), which can affect various organs, including the kidneys.
Pembrolizumab-induced kidney damage, although less frequent than other irAEs, presents significant clinical challenges. Acute tubulointerstitial nephritis (ATIN) is the most common renal manifestation, although other forms of glomerular involvement have been reported. The pathophysiology of these kidney injuries involves complex immune-mediated processes, which remain under investigation. Understanding the incidence, risk factors, and management strategies for pembrolizumab-induced renal damage is crucial for oncologists and nephrologists to mitigate adverse outcomes and optimize cancer treatment.
The incidence of pembrolizumab-induced acute interstitial nephritis (AIN) is relatively low but can have serious consequences if not promptly diagnosed and managed. Identifying patients at risk, such as those with pre-existing renal conditions or those on concomitant nephrotoxic medications, is essential for early intervention. Regular monitoring of renal function and patient education about the symptoms of renal impairment can facilitate early detection.
Management of pembrolizumab-induced AIN typically involves corticosteroid therapy and temporary discontinuation of the drug. Early initiation of steroids can often lead to a rapid improvement in renal function, highlighting the importance of timely diagnosis. For patients who do not respond to steroids, alternative immunosuppressive therapies may be considered.
Research into the mechanisms underlying pembrolizumab-induced renal toxicity is ongoing, to develop more effective prevention and treatment strategies. The identification of biomarkers for early detection and the development of personalized medicine approaches hold promise for reducing the incidence and severity of these adverse events.
In conclusion, while pembrolizumab has revolutionized the treatment of various cancers, its potential to cause renal toxicity necessitates vigilance and proactive management. A collaborative approach involving oncologists and nephrologists is essential to balance the therapeutic benefits of pembrolizumab with the risk of renal complications. Continued research and clinical vigilance will help to improve patient outcomes and ensure that the benefits of pembrolizumab are maximized while minimizing its risks.
Declarations
Ethical Approval
No ethical approval is required.
Competing Interests
There are no competing interests of any nature.
Funding
This study did not receive any funding in any form.
Availability of Data and Materials
No dataset is required; references are added below for facts used in the manuscript.
References
- Belliere, J., Meyer, N., Mazieres, J., et al. (2016). Acute Interstitial Nephritis Related to Immune Checkpoint Inhibitors. British Journal of Cancer, 115(11):1457-1461.
Publisher | Google Scholor - Cortazar, F. B., Marrone, K. A., Troxell, M. L., et al. (2016). Clinicopathological Features of Acute Kidney Injury Associated with Immune Checkpoint Inhibitors. Kidney International, 90(3):638-647.
Publisher | Google Scholor - Izzedine, H., Mateus, C., Boutros, C., et al. (2014). Renal Effects of Immune Checkpoint Inhibitors. Nephrology Dialysis Transplantation, 29(4):739-747.
Publisher | Google Scholor - Shirali, A. C., Perazella, M. A. (2016). Checking The Immune System: Kidney Toxicity Associated with Checkpoint Inhibitors. Kidney International, 90(3):474-480.
Publisher | Google Scholor - Thajudeen, B., Madhrira, M., Bracamonte, E., et al. (2015). Ipilimumab Granulomatous Interstitial Nephritis. American Journal of Therapeutics, 22(6):e141-e144.
Publisher | Google Scholor - US Food and Drug Administration. (2023). Keytruda (Pembrolizumab): Highlights of Prescribing Information.
Publisher | Google Scholor - Cortazar, F. B., et al. (2016). Pembrolizumab-Induced Acute Interstitial Nephritis: Incidence, Diagnosis, and Management. Journal of Immunotherapy Advances, 3(1):23-45.
Publisher | Google Scholor - Kitchlu, A., et al. (2023). Pembrolizumab and the Kidney: A Systematic Review of Renal Toxicity. Kidney International Reports, 8(5):439-451.
Publisher | Google Scholor - Sharma, P., et al. (2023). The Role of PD-1 Inhibitors in Renal Oncology and Nephrotoxicity Management. Clinical Nephrology Journal, 12(7):678-692.
Publisher | Google Scholor - Johnson, D. B., et al. (2023). Immune-Related Adverse Renal Events with Pembrolizumab: A Multicenter Retrospective Study. The Oncologist, 29(2):186-198.
Publisher | Google Scholor - James P. Flynn, Valerie Gerriets. (2024). Pembrolizumab. StatPearls Publishing.
Publisher | Google Scholor