Research Article
Outcomes With Optimal Treatment in Geriatric Head and Neck Cancers – Tertiary Cancer Centre Experience
1Department of Radiation Oncology, JNMC and KLES Dr Prabhakar Kore Hospital & MRC, KAHER, Belgaum, India.
2Medical Oncology, JNMC and KLES Dr Prabhakar Kore Hospital & MRC, KAHER, Belgaum, India.
3Surgical Oncology, JNMC and KLES Dr Prabhakar Kore Hospital & MRC, KAHER, Belgaum, India.
*Corresponding Author: Imtiaz Ahmed, Department of Radiation Oncology, JNMC and KLES Dr Prabhakar Kore Hospital & MRC, Belgaum, India.
Citation: I Ahmed, S Krishnamurthy, R Bhise, K Vinchurkar, M Kalloli. (2022). Outcomes With Optimal Treatment in Geriatric Head and Neck Cancers-Tertiary Cancer Centre Experience. International Journal of Clinical and Molecular Oncology, BRS Publishers. 1(1); DOI: 10.59657/2993-0197.brs.22.001
Copyright: © 2022 Imtiaz Ahmed, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: August 25, 2022 | Accepted: September 23, 2022 | Published: September 29, 2022
Abstract
Aim: Older patients with locally advanced head and neck cancers (LA-HNC) are under-represented in clinical trials and denied standard treatment with concurrent Chemo-radiation. Most are treated with Radiotherapy (RT) alone. However, with the use of Intensity Modulated Radiation Therapy (IMRT) and good supportive care, even this cohort of patients can be considered for chemo-radiation.
Methods and Materials: 69 patients with age >65 years with LA-HNC treated between April 2015 and December 2019 in our Institute were prospectively evaluated for treatment compliance and outcomes. All patients were planned to receive 70Gy in 33-35 fractions with IMRT and weekly Cisplatin at a dose of 40mg/m2 (or Carboplatin-AUC-2). Loco-regional Control (LRC), Overall survival (OS) and prognostic factors were evaluated.
Results: Median age at presentation was 67 years (65-81). 54 were male. 64% had Karnofsky Performance Status of >90. 42% had Oropharyngeal Primary. 17% had co-morbidities. 66% had T3 disease, 77% had Node positive disease. 54% had stage III disease. All patients completed 70Gy. 81% patients received at least 5 (>200mg/m2) chemotherapy cycles. Acute Grade3 toxicity was seen in 20% of patients. 64% had complete response. With a median follow up of 23.6 months (3-71), OS was 53.5%. Estimated 2-year LRC was 60%; estimated 2- and 5-year OS was 53.5% and 34.3% respectively. On Univariate analysis, age <70 years, Cisplatin use, limited nodal disease, Stage III and complete response to treatment showed good OS (p<0.05).
Conclusion: Definitive chemo-IMRT approach in older LA-HNC is well tolerated with good clinical outcomes. Hence older age should not be a barrier for standard treatment.
Keywords: chemoradiation; geriatric; head and neck cancers
Introduction
The proportion of older patients with cancer is increasing with increase in life expectancy. Treating these patients with cancer poses varied and unique challenges. In locally advanced head and neck cancer (LA-HNC), multidisciplinary treatment with cisplatin based Concurrent Chemo-Radiation (CCRT) is the current standard of care [1]. It is thought that because of declining physical strength, compromised functional ability, associated co-morbidities and poor performance status, these patients might be vulnerable to the toxicities associated with standard treatment and thus treatment intolerance. Furthermore, the development of late toxicities such as dysphagia and feeding-tube dependence can have a long-term detrimental effect. Thus, may limit the use of aggressive therapy and many are treated with Radiotherapy (RT) alone [2]. Most of the older patients are excluded from the clinical trials [3] and hence there is no robust clinical evidence to practice or extrapolate the current standard treatment to this cohort of patients. However, the chronological age by virtue of date of birth is a poor surrogate marker of overall performance status and denial of standard treatment to these patients cannot be substantiated.
Treatment with Intensity Modulated Radiation Therapy (IMRT) is the basic approach in present day RT practice, and has shown to reduce toxicity compared to conventional RT [4]. It is seen that concurrent chemotherapy with weekly Cisplatin is better tolerated than 3 weekly Cisplatin more and is widely practiced in Indian sub-continent [5]. Along with good supportive care, Chemoradiation with IMRT and weekly Cisplatin in older patients seems feasible.
We prospectively evaluated compliance to treatment, loco-regional control (LRC) and overall survival (OS) in older patients with LA-HNC treated with definitive IMRT and concurrent weekly cisplatin.
Subject and Methods
After taking permission from institute review board, we prospectively studied older patients with age > 65 years having locally advanced carcinoma of oropharynx, hypopharynx or larynx, planned for definitive chemoradiation at our institution.
Inclusion criteria: histologically proven squamous cell carcinoma, stage III-IVB, age >/= 65years, Karnofsky Performance Status (KPS) >/= 70, normal complete blood counts, normal renal function test (RFT), controlled co-morbid conditions.
Exclusion criteria: age less than 65years KPS less than 70, deranged RFT, uncontrolled co-morbid conditions, stage IV C disease.
All eligible patients underwent clinical evaluation and endoscopy. Contrast Enhanced Computed Tomography (CECT) of Neck was done for all patients for locoregional staging. Chest X ray was done as a part of metastatic work-up. Baseline CBC, RFT and weight were noted; need for feeding procedure or tracheostomy was assessed. All patients were staged as per AJCC-TNM-2007 7th edition staging [6].
Written and informed consent was obtained before treatment. All patients underwent dental examination before chemoradiation.
Radiotherapy:
All patients were planned with IMRT technique. Patients were immobilised with 4 clamp head and neck thermoplastic mask followed by CECT simulation with 2.5 mm slice thickness. Critical normal structures and Planning Target volumes (PTV) – PTV high risk receiving 70Gy (PTV-70), PTV intermediate risk receiving 59.4 Gy (PTV-59.4) and PTV low risk receiving 54-56Gy (PTV-54) in 33-35 fractions over 6.5 -7 weeks were defined and planned with Eclipse version 11 treatment planning system using 7 or 9 field IMRT.
Chemotherapy:
Weekly Cisplatin at a dose of 40mg/m2 was administered concurrent with radiation. Weekly Carboplatin at a dose of Area under the Curve (AUC) - 2 was also allowed in patients with serum creatinine more than 1.4mg/dl.
Toxicity and Response assessment:
Acute haematological and non-haematological toxicities during the course of radiation were assessed according to RTOG/EORTC [7] acute toxicity grading – every week, at the end of treatment, 1 month and 3-month post treatment.
Weight loss, need for supportive care and treatment breaks during radiation were documented. Response assessment was done with CECT neck/WBPET-CT at 3 months post treatment and assessed according to response evaluation criteria in solid tumours (RECIST) criteria version 1.1 [8].
Statistics:
SPSS version 16 software was used for statistical analysis. Overall survival (OS) was defined as the time between the dates of start of treatment to the date of death/last seen in clinic/last telephonic information. Loco-regional control (LRC) was defined as the time between the dates of start of treatment to the date of local or regional recurrences; in patients who did not achieve complete response (CR) it was taken as failure at the time of assessment. Kaplan-Meier estimates were performed to calculate the OS and LRC. Univariate analysis with log rank test was performed on potential prognostic factors affecting the survival. P value of less than 0.005 was considered statistically significant. Those prognostic factors with significant p value on univariate analysis were further evaluated with multivariate analysis using Cox Regression model.
Results
We prospectively evaluated 69 older patients with LA-HNC planned for definitive chemo-IMRT treated between April 2015 and December 2019.
Median age at presentation was 67 years (range - 65-81 years), 22 (32%) patients were aged more than 70 years; 54 (78%) were male and 44 (64%) had KPS of 90. Twelve (17%) had co-morbid conditions. Twenty-nine (42%) had Oropharyngeal and 27 (39%) had Hypopharyngeal primaries. Forty-six (66%) had T3, 53 (77%) had Node positive disease, and 37 (54%) had stage III disease. Demographic and tumour characteristics are shown in table-1.
| Characteristic | Number = 69 |
| Age (Years) | |
| Median | 67 (range 65-81) |
| <70>70 | 47/22 |
| Sex | |
| Male/Female | 54/15 |
| Co-morbidities | 12 |
| KPS | |
| 90/80/70 | 44/21/4 |
| Baseline Hemoglobin (gm/dl) | |
| Mean | 12 (range 7.5-18) |
| Baseline Weight (Kgs) | |
| Mean | 49 (range 29-86) |
| Baseline Feeding Tube | 5 |
| Tracheostomy | 3 |
| Site of Primary | |
| Oropharynx | 29 |
| Hypopharynx | 27 |
| Larynx | 13 |
| Tumour Stage | |
| T2/T3/T4a | 14/46/8 |
| Nodal Stage | |
| N0/N1 | 16/23 |
| N2/N3 | 27/03 |
| Stage Group | |
| III | 37 |
| IVA | 32 |
| Chemotherapy | |
| Cisplatin | 44 |
| Carboplatin | 25 |
| Chemotherapy (cycles) | |
| Median | 6 (IQR 5-6) |
| 5 cycles or more (>200mg/m2) | 56 |
| OTT (days) | |
| Median | 50 (range 42-65) |
| Weight Loss | |
| Mean | 8.7% |
Table 1: Patient characteristics
Treatment Compliance and Toxicity:
All patients (100%) completed the planned RT (70Gy in 33/35 fractions). 83% of patients received at least five or more chemotherapy cycles with a median of six cycles. Forty-four (64%) received Cisplatin and 25 (36%) received Carboplatin; 81% received a cumulative Cisplatin dose of >200mg/m2.
Mean overall treatment time (OTT) was 50 days (range 42-65 days). Ten (14.5%) patients had treatment break of more than five days (range 6-10) - one patient for emergency tracheostomy, three patients for grade-3 thrombocytopenia and three patients for grade-3 neutropenia which were self-limiting; one had poor oral intake, one had pneumonia and one had pyrexia of unknown origin, all three required in-patient management. Average weight loss was 8.7%, two patients gained weight.
Grade-3 neutropenia was seen in four patients (5.7%), Grade-3 thrombocytopenia was seen in three patients (4.3%). All seven of these patients recovered without any intervention. Grade-3 oral mucositis, pharyngitis and laryngitis were seen in eleven patients (16%), ten patients (14.4%), and three patients (4.3%) respectively.
Treatment Response and Survival:
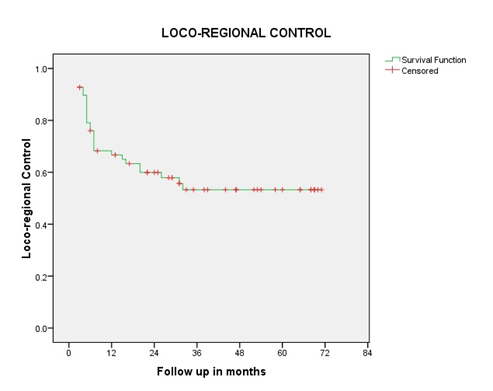
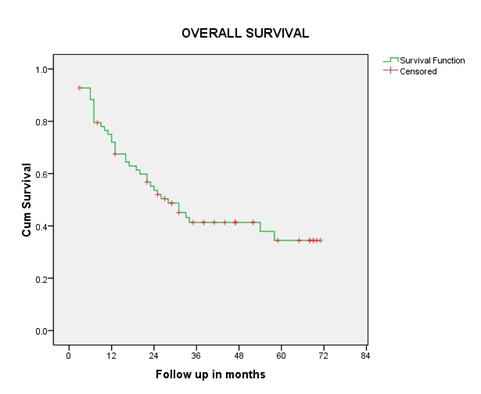
Overall response rate was 97%, 44 patients (64%) had Complete Response (CR) and 23 patients (33.3%) had Partial Response (PR), one had Progressive Disease (PD), and one had Stable Disease (SD). CR rates were 77% in patients less than 70 years. At the last follow up, 28 patients are alive and disease free and one patient is alive with disease. With an overall median follow up of 23.6 months and 47 months in surviving patients (range 3-71 months) the median OS was 53.5%. The estimated 2-year, 3-year, and 5-year OS were 53.5%, 41.3% and 34.3% respectively for the overall population. The 2-year LRC was 60%. The Kaplan Meir- survival curve for OS and LRC is shown in figure 1and 2. Stage and site-specific survival is shown in table 2.
| Site | Stage | No of Pts | Median OS | 2-year OS | P value |
| Oropharynx | III | 13 | 54 | 73.8% | 0.04 |
| IV | 16 | 13 | 35.7% | ||
| Hypopharynx | III | 13 | 25 | 53.8% | 0.94 |
| IV | 14 | 22 | 42.9% | ||
| Larynx | III | 10 | NR | 70% | 0.78 |
| IV | 03 | NR | 66.7% |
Table 2: Site and stage specific survival
Prognostic Factors:
On univariate analysis of the potential prognostic factors, age less than 70years, N0-N1 nodal group (low nodal burden), Stage III disease, use of Cisplatin chemotherapy and CR to treatment showed good OS with statistically significant p value (<0>
| Variable | Median Overall Survival (months) | P value | |
| Age (years) < 70>70 | 33.9 | 10.3 | 0.023 |
| N Status N0-N1 vs N2/3 | 54.4 | 19.4 | 0.05 |
| Stage Group III vs IV | 54.4 | 19.6 | 0.056 |
| Chemo Drug Cisplatin vs Carboplatin | 33.9 | 17.3 | .019 |
| Response CR vs Others | Not Reached | 10.7 | 0.00 |
Table 3: Univariate analysis of prognostic factor.
On multivariate analysis, patients with age less than 70 years showed significant median OS benefit - 33.9 v/s 10.3 months (p=0.023). Patients who had CR to treatment fared better as compared to patients who did not - median OS was not reached v/s 10.7 months (p=0.00).
We further evaluated for any OS difference with respect to Site and Stage in patients < 70> 70 years. Median OS for Oropharynx, Larynx and Hypopharynx in patients < 70> 70 years were 26 v/s 13months (p=0.46); not reached v/s 17months (p=0.18) and 31 v/s 7months (p=0.048) respectively. However, there was no difference when stratified by stage in < 70> 70 years, 58 vs 17months (p=0.163) for stage III and 23 v/s 10months (p=0.158) for stage IV respectively.
We further evaluated for any OS difference with respect to Site and Stage in patients < 70> 70 years. Median OS for Oropharynx, Larynx and Hypopharynx in patients < 70> 70 years were 26 v/s 13months (p=0.46); not reached v/s 17months (p=0.18) and 31 v/s 7months (p=0.048) respectively. However, there was no difference when stratified by stage in < 70> 70 years, 58 vs 17months (p=0.163) for stage III and 23 v/s 10months (p=0.158) for stage IV respectively.
We further evaluated for any OS difference with respect to Site and Stage in patients < 70> 70 years. Median OS for Oropharynx, Larynx and Hypopharynx in patients < 70> 70 years were 26 v/s 13months (p=0.46); not reached v/s 17months (p=0.18) and 31 v/s 7months (p=0.048) respectively. However, there was no difference when stratified by stage in < 70> 70 years, 58 vs 17months (p=0.163) for stage III and 23 v/s 10months (p=0.158) for stage IV respectively.
Patterns of Failure:
Of the 44 patients who had CR, 17 patients have expired. Of the 17 patients, 12 expired without evidence of recurrence. Two patients developed second primaries after 2-5 years post treatment - one had carcinoma of oesophagus treated with chemoradiation, one had carcinoma of tongue treated with re-irradiation. Two had distal only recurrence (bone and lung) treated with palliative Chemotherapy and palliative RT to site of recurrence; only one patient had local recurrence. All five patients subsequently succumbed to disease.
Of the 25 patients who did not achieve CR, only one patient was of laryngeal primary. All patients (100%) had local and nine had loco-regional recurrence. One patient received salvage chemotherapy – Paclitaxel and Carboplatin and is alive without disease and one patient is on Gefitinib and is alive with disease. None of the other 23 patients received any form of salvage treatment and have expired.
Late Toxicity:
Of the 29 alive patients, 13 did not report any late toxicity, four had spicy intolerance, four had grade-2 xerostomia, one had grade-1 peripheral neuropathy (patient who received salvage Chemotherapy). Late toxicity was not documented in nine patients. No patients had tube dependency. Post treatment, feeding and tracheostomy tubes were removed in all patients. None reported any long term renal or oto toxicity.
Discussion
Although older patients constitute a significant portion of locally advanced head and neck cancers, patterns of care have shown that they are considered for less aggressive treatment with RT alone when compared to their younger counterparts, with only 25% receiving chemoradiation [9]. In our study, all patients (100%) completed planned RT (70Gy) and 62 patients (90%) received at least four cycles of Cisplatin and 83% at least five cycles; reflecting good compliance to chemoradiation in older patients.
Increased OTT and treatment interruptions due to associated toxicities like neutropenia, mucositis, dysphagia, poor nutritional status, and increased susceptibility to infections result in poor local control. In our study, acute grade-3 neutropenia, dysphagia and oral mucositis were seen in 5.7%, 14.4%, and 16% respectively with a median OTT of 50 days (range 42-62 days) which are quite acceptable as against that reported by Nguyen et al [10] where 59% of older patients treated with chemoradiation had grade 3-4 mucositis and 26% had grade 3-4 hematologic toxicity.
The 2-year LRC in our study was 60% and 2-year Overall Survival (OS) was 53.5%, similar to the 2-year OS rate of 48% quoted by National Cancer Data Base of US [11], in older patients treated with chemoradiation compared to 38% in patients treated with RT alone. Hence there is a definite survival benefit with the addition of concurrent chemotherapy to RT in older patients with LA-HNC.
Most of the recent trials of chemoradiation in head and neck cancer [12,13,14], have significant number of Human Papilloma Virus (HPV) positive patients (>70% of Oropharyngeal cancers) where the 5-year OS are in the range of 75-85%. It has to be noted that, HPV positive patients are demographically and biologically different from older patients who are mostly HPV negative. The estimated 5-year OS in our study was 34.3% which is comparable to 30% as quoted by National Cancer Data Base of US [11] and other similar retrospective Institutional studies [15,16,17] where all patients were older.
The overall response rate in our study was 97% (67 patients), with 66% (44 patients) achieving CR which are comparable to the current evidence for CCRT [12,13] and definitely better than the results of treatment with RT alone. Univariate and multivariate analysis in our study showed that, achieving CR was the most important prognostic factor for survival. Hence, when older patients are treated with RT alone where the response rates are poor definitely their survival is also compromised.
Quality of Life (QOL) post chemoradiation is a very important aspect of treatment outcomes especially in older patients. Though no questionnaire based QOL evaluation was done in our study, of the 29 patients alive, 45% (13 patients) did not report any late toxicity. Even in those who reported late toxicity, none reported grade-3 toxicity and none had tube dependency.
Iternational Society of Geriatric Oncology (SIOG) [18] suggests that, aggressive combined modality treatment is appropriate where co-morbidities permit and recommend the use of IMRT or other highly conformal techniques to reduce acute and late toxicity in older patients. All patients in our study were treated with IMRT and is definitely one of the prime factors contributing to good compliance to treatment.
With clear evidence supporting CCRT, improvements in the safety of the delivery of chemotherapy and reduction in radiation toxicity due to the use of IMRT, there is a trend towards use of chemotherapy in older patients with LA-HNC and there is a gradual change in practice since 2004 to 2012 in this regard [19].
While Concurrent 3-weekly 100 mg/m2 Cisplatin is considered standard, meta-analysis of 3-weekly v/s weekly Cisplatin by Petr Szturz [20] failed to show any survival difference (5-year OS of 40%) and weekly regimen was more compliant with less toxicity especially in the definitive setting. While the overall grade 3 toxicity in our study was 20% which used weekly Cisplatin, the corresponding figures for the same are in the range of 74-80% in the RTOG [12,13] studies using 3 weekly 100mg/m2 of Cisplatin.
Combined use of IMRT and weekly Cisplatin is better tolerated with good outcomes and thus is a reasonably good combination in older patients with LA-HNC.
Though MACH-NC [1] showed no overall survival benefit in patients with age more than 70 years, non-Cancer related Deaths (NCRD) may have led to this dilutional effect. Secondly, this population consisted only 4% (693) of the total cases (17,346) in the analysis, probably limiting the statistical power. In our study too, patients in the sub-strata of age more than 70 years had dismal survival. The average life expectancy in India is 69.66 years [21], hence NCRD might be the likely explanation in our study too; also, patient number is less to conclusively evaluate the reason. Survival patterns in older patients with head and neck cancer have also shown the same trends where the 5-year OS and NCRD rates were significantly worse for older patients than for young patients – 45.5% v/s 58.2% and 39% v/s 20.7% respectively [22]
One of the drawbacks of our study is that, the recommended screening tools for assessing vulnerability in older adults with cancer - like Geriatric – 8 score [23] or any other similar scores were not used, though most of the parameters included in the Geriatric-8 score were taken into consideration (performance status, co-morbidities, baseline weight). Currently the evidence for treatment in older patients with LA-HNC is more of retrospective, single institution experiences, where patients have been non-uniformly treated with – RT alone or using Cetuximab, including oral cavity tumours and those who received adjuvant RT with selection and treatment bias [15,16,17]. Though the patient number is less, the results of our study are very important in current practice as ours is one of the very few prospectively conducted studies from South East Asian Region in a cohort of uniformly treated patient population whereby the interpretation of the results is easy and meaningful.
Conclusion
Definitive IMRT and concurrent weekly chemotherapy is a feasible treatment option for older patients with LA-HNC with good clinical outcomes and acceptable toxicities. It should be considered the standard in Geriatric patients with good performance status, without any co-morbidities or well controlled co-morbid conditions.
Conflict of Interests
Nil
References
- Lacas B, Carmel A, Landais C, Wong SJ, Licitra L, Tobias JS et al. (2021). MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 107 randomized trials and 19,805 patients, on behalf of MACH-NC Group. Radiother Oncol. 156:281-293.
Publisher | Google Scholor - Juarez JE, Choi J, St John M, Abemayor E, TenNapel M and Chen MA. (2017). Patterns of care for elderly patients with locally advanced head and neck cancer. Int J Radiation Oncol Biol Phys. 98(4):767-774.
Publisher | Google Scholor - Murthy VH, Krumholz HM, Gross CP. (2004). Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 291:2720-2726
Publisher | Google Scholor - Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C et al. (2011). Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 12:127-136.
Publisher | Google Scholor - Goyal G, Patil VM, Noronha V, Joshi A, Khaddar S, Kakkar S et al. (2018). Once-a-week versus once-every-3-weeks cisplatin in patients receiving chemoradiation for locally advanced head-and-neck cancer: A survey of practice in India. Cancer Res Stat Treat. 1:63-67.
Publisher | Google Scholor - American Joint Committee on Cancer. AJCC cancer staging handbook, 7th edn. Springer: New York; 2010.
Publisher | Google Scholor - Cox JD, Stetz J and Pajak TF. (1995). Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int. J. Radiation Oncology Biol. Phys. 31:1341-1346.
Publisher | Google Scholor - Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al. (2009). New Response Evaluation Criteria in Solid Tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 45:228-247.
Publisher | Google Scholor - VanderWalde NA, Meyer AM, Deal AM, Layton JB, Liu H, Carpenter WR et al. (2014). Effectiveness of Chemoradiation for head and neck cancer in an older patient population. Int J Radiation Oncol Biol Phys. 89(1):30-37.
Publisher | Google Scholor - Nyugen NP, Vock J, Chi A, Vinh-Hung V, Dutta S, Ewell L et al. (2012). Impact of intensity-modulated and image-guided radiotherapy on elderly patients undergoing chemoradiation for locally advanced head and neck cancer. Strahlenther Onkol. 188:677-685.
Publisher | Google Scholor - Amini A, Jones BL, McDermott JD, Serracino HS, Jimeno A, Raben D et al. (2016). Survival outcomes with concurrent chemoradiation for elderly patients with locally advanced head and neck cancer according to the National Cancer Data Base. Cancer. 122(10):1533-1543.
Publisher | Google Scholor - Nguyen-Tan PF, Zhang Q, Ang KK, Weber RS, Rosenthal DI, Soulieres D et al. Randomized Phase III Trial to Test Accelerated Versus Standard Fractionation in Combination with Concurrent Cisplatin for Head and Neck Carcinomas in the Radiation Therapy Oncology Group 0129 Trial: Long-Term Report of Efficacy and Toxicity. J ClinOncol 32:3858-3867
Publisher | Google Scholor - Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS et al. Randomized Phase III Trial of Concurrent Accelerated Radiation Plus Cisplatin with or Without Cetuximab for Stage III to IV Head and Neck Carcinoma: RTOG 0522. J ClinOncol 32:2940-2950.
Publisher | Google Scholor - Gebre-Medhin M, Brun E, Engstrom P, Cange HH, Hammarstedt-Nordenvall L, Reizenstein J et al. (2020). ARTSCAN III: A Randomized Phase III Study Comparing Chemoradiotherapy with Cisplatin Versus Cetuximab in Patients with Locoregionally Advanced Head and Neck Squamous Cell Cancer. J ClinOncol. 39:38-47.
Publisher | Google Scholor - Viani GA, Faustino AC, Danelichen AFB, Matsuura FK, Neves LVF, Fernandes MH et al. (2021). Radiotherapy for locally advanced head and neck cancer in elderly patients: results and prognostic factors a single cohort. Rep Pract Oncol Radiother. 26(1):12-19.
Publisher | Google Scholor - Haehl E, Rühle A, David H, Kalckreuth T, Sprave T, Stoian R et al.et al. (2020). Radiotherapy for geriatric head-and-neck cancer patients: what is the value of standard treatment in the elderly? Radiat Oncol. 15: 31.
Publisher | Google Scholor - Müller von der Grün J, Martin D, Stöver T, Ghanaati S, Rödel C, Balermpas P. (2018). Chemoradiotherapy as Definitive Treatment for Elderly Patients with Head and Neck Cancer. Biomed Res Int.
Publisher | Google Scholor - Kunkler IH, Audisio R, Belkacemi Y, Betz M, Gore E, Hoffe S et al. (2014). SIOG Radiotherapy Task Force. Review of current best practice and priorities for research in radiation oncology for elderly patients with cancer: the International Society of Geriatric Oncology (SIOG) task force. Ann Oncol. 25(11):2134-2146.
Publisher | Google Scholor - Ward MC, Reddy CA, Adelstein DJ, Koyfman SA. (2016). Use of systemic therapy with definitive radiotherapy for elderly patients with head and neck cancer. A National Cancer Data Base analysis. Cancer. 122:3472-3483.
Publisher | Google Scholor - Szturz P, Wouters K, Kiyota N, Tahara M, Prabhash K, Noronha V et al. (2017). Weekly Low-Dose Versus Three-Weekly High-Dose Cisplatin for Concurrent Chemoradiation in Locoregionally Advanced Non-Nasopharyngeal Head and Neck Cancer: A Systematic Review and Meta-Analysis of Aggregate Data. Oncologist. 22(9):1056-1066.
Publisher | Google Scholor - The World Bank. (2019) Life expectancy at birth, total (years) – India.
Publisher | Google Scholor - Sommers LW, Steenbakkers RJHM, Bijl HP, Vemer-van den Hoek JGM, Roodenburg JLN, Oosting SF et al. (2017). Survival patterns in elderly head and neck squamous cell carcinoma patients treated with definitive radiation therapy. Int J Radiot Oncol Biol Phys. 98(4):793-801.
Publisher | Google Scholor - Kenis C, Decoster L, Puyvelde KV, De Grève J, Conings G, Milisen K et al. (2014). Performance of Two Geriatric Screening Tools in Older Patients with Cancer. Journal of Clinical Oncology. 32(1):19-26.
Publisher | Google Scholor