Case Report
Osteoporosis and Stress Fractures in a 9-year-old? Musculoskeletal Findings in Severe Combined Immunodeficiency
1Radiology, Nova Southeastern University Dr. Kiran C. Patel College of Osteopathic Medicine, Davie, USA.
2Radiology, Aventura Hospital and Medical Center, Hollywood, USA.
3Radiology, Cincinnati Children's Hospital Medical Center, Cincinnati, USA.
4Radiology, Aventura Hospital and Medical Center, Aventura, USA
*Corresponding Author: Sana Padival, Radiology, Nova Southeastern University Dr. Kiran C. Patel College of Osteopathic Medicine, Davie, USA.
Citation: Padival S., Montgomery T.P., Oestreich A., Banks J. (2024). Osteoporosis and Stress Fractures in a 9-year-old? Musculoskeletal Findings in Severe Combined Immunodeficiency. Clinical Case Reports and Studies, BioRes Scientia Publishers. 5(4):1-03. DOI: 10.59657/2837-2565.brs.24.125
Copyright: © 2024 Sana Padival, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: March 29, 2024 | Accepted: April 11, 2024 | Published: April 18, 2024
Abstract
Omenn Syndrome (OS) is an autosomal recessive and potentially fatal variant of severe combined immunodeficiency (SCID). The musculoskeletal ramifications of OS are not commonly considered. A 9-year-old patient with OS presented with foot and hip pain and underwent radiographic evaluation, revealing prominent vertical trabeculae and acetabular sclerosis, consistent with osteopenic changes. Ankle radiograph demonstrated stress fracture and physeal injury. There was no evidence of soft tissue trauma or non-traumatic injury. Given the history of OS and chronic immunotherapy treatment, these radiographic findings were presumed to be secondary to the patient’s OS. Musculoskeletal manifestations of OS have great potential for affecting a patient’s quality of life and long-term bone health through decreased bone mineral density, pain due to stress fracture, and potentially pathologic fracture. Further understanding of OS’s influence on osteoimmunology will improve the ability to preserve a patient’s bone mineral density and quality of life.
Keywords: omenn syndrome; osteoporosis; stress fractures
Introduction
Omenn Syndrome (OS) is an autosomal recessive and potentially fatal variant of severe combined immunodeficiency (SCID). The median age of symptomatic onset is four weeks, including erythematous rash, hepatosplenomegaly, lymphadenopathy, recurrent infections and occasionally alopecia. Leukocytosis is frequently observed due to eosinophilia and lymphocytosis [1]. If bone marrow or cord blood stem cell transplantation is not performed, OS is typically fatal by six months of age due to recurrent life-threatening infections.
The case presented highlights the musculoskeletal ramifications of OS, which are not commonly associated with the condition. These include bone demineralization and stress fractures possibly related to the underlying immunologic abnormalities of OS patients. The case and discussion herein, presents a review of the literature, which suggests that autoreactive T cells in the bone marrow can stimulate osteoclast maturation. It also opens the possibility of using osteoporosis treatments for such complications and stresses the importance of studying the mechanisms responsible for bone loss.
Case Presentation
A 9-year-old patient with prior diagnosis of OS presented with foot and hip pain and underwent radiographic evaluation of the lower extremities. Hip and foot radiographs revealed prominent vertical trabeculae and acetabular sclerosis, consistent with osteopenic and osteoarthritic changes (Figures 1, 2). Ankle radiograph demonstrated chronic distal tibial and fibular physeal injuries in keeping with stress fractures (Figure 3). There was no radiographic evidence of soft tissue trauma or non-traumatic injury. Given the history of OS and chronic immunotherapy treatment, the osteopenia and stress fractures were presumed to be secondary to the patient’s OS and chronic immunosuppressive therapy. Subsequent follow-up reportedly showed no change in the patient's symptoms or radiographic findings.
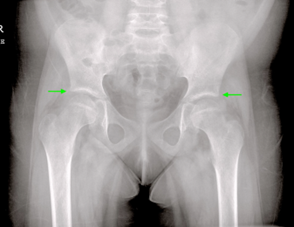
Figure 1: Pelvis Radiograph
Anterior-posterior radiograph of the pelvis demonstrates bilateral superior acetabular sclerosis (green arrows), consistent osteoarthritic changes of the femoro-acetabular joints, which is atypical in a healthy pediatric patient.
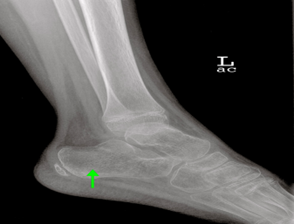
Figure 2: Lateral Ankle Radiograph
Lateral ankle radiograph demonstrates prominent calcaneal trabeculae (green arrow), consistent with osteopenia, which is also atypical in a healthy pediatric patient.
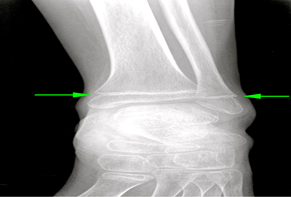
Figure 3: Anterior-Posterior Ankle Radiograph
Anterior-Posterior ankle radiograph demonstrates narrowing and sclerosis of the tibial and fibular physes (green arrow), consistent with physeal injury. Although common in the pediatric population, this was thought to represent pathologic stress fracture in the setting of diffuse osteopenia.
Discussion
OS is a genetic disorder and potentially fatal form of SCID. To contrast the two, classical SCID results in complete absence of T-cells and B-cells [1,2]. Comparatively, OS has been associated with residual T-cell activity, leading to oligoclonal T-cell expansion [2-4]. This T-cell expansion in OS often has associated inflammatory changes and cellular damage associated with overproduction of cytokines. The T-cell clones, particularly T-helper 2 cells (Th2), secrete cytokines such as Interleukin (IL) -13, IL-5, and IL-4, which are mainly responsible for producing inflammation and inducing autoimmunity [5]. Other cytokines such as IL- 1, IL-1, IL-17, and Tumor Necrosis Factor-alpha can be produced [5], which are known contributors to the bone rebuilding phase in fracture healing, suggesting that the T-cells and subsequent IL's play a critical role in bone homeostasis. These inappropriately activated T-cells can also produce nuclear factor kappa light chain (NF-κB) and receptor activator NF-κB ligand (RANKL), which is known to bind to its Receptor Activator for NF-κB (RANK) which is found on the surface of osteoclasts, T-cells, and some stromal cells [6,7]. This can regulate osteoclast differentiation and maturation, eventually leading to bone demineralization and density loss.
Given the patient's history of OS and the resultant increase in autoreactive T-cells, it is possible that the patient suffered from osteoporotic manifestations due to RANKL expression. When autoreactive T-cells home back to the bone marrow from the periphery, osteoclastogenesis is triggered, which further triggers osteoclastic bone resorption when RANKL binds to RANK [7]. Additionally, treatments utilized in OS, such as glucocorticoids, are known to induce osteoclastogenesis through the same T-cell mediated mechanisms. Preventing osteoclast-mediated bone loss in patients with OS could potentially be achieved by inhibiting the accumulation of T-cells in the bone marrow by using anti-chemotactic agents, anti-resorptive drugs such as anti-RANKL mediators, or anabolic drugs to increase osteoblast activity [8]. Further basic and clinical research is needed to fully understand the role of osteoimmunology in OS in order to address the musculoskeletal pathology as seen in our patient.
Conclusion
Musculoskeletal manifestations of Omenn Syndrome are often overlooked but have great potential for affecting a patient’s quality of life and long-term bone health through decreased bone mineral density, pain due to stress fracture, and potentially pathologic fracture. Further understanding of OS’s influence on osteoimmunology will improve the ability of clinicians to provide targeted therapies to preserve patient’s bone mineral density and quality of life.
Additional Information
Disclosures
Human subjects: Consent was obtained or waived by all participants in this study.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
References
- Aleman K, Noordzij JG, de Groot R, et al. (2001). Reviewing Omenn Syndrome. Eur J Pediatr., 160:718-725.
Publisher | Google Scholor - Niehues T, Perez-Becker R, Schuetz C. (2010). More than just SCID--the phenotypic range of combined immunodeficiencies associated with mutations in the recombinase activating genes (RAG) 1 and 2. Clin Immunol. 135:183-192.
Publisher | Google Scholor - Grunebaum E, Bates A, Roifman CM. (2008). Omenn syndrome is associated with mutations in DNA ligase IV . J Allergy Clin Immunol., 122:1219-1220.
Publisher | Google Scholor - Yachie A. (2017). Omenn: Syndrome and DNA recombination defects. Nihon Rinsho Meneki Gakkai Kaishi., 40:179-189.
Publisher | Google Scholor - Chaim M. Roifman, Eyal Grunebaum, (2013). 35 - Primary T-cell immunodeficiencies, Clinical Immunology (Fourth Edition). Elsevier, 437:453.
Publisher | Google Scholor - Song L, Cao L, Liu R, et al. (2020). The critical role of T cells in glucocorticoid-induced osteoporosis. Cell Death Dis. 14:45.
Publisher | Google Scholor - von Gunten S, Simon HU. (2020) Linking glucocorticoid-induced osteoporosis to osteoimmunology. Cell Death Dis., 14:11.
Publisher | Google Scholor - Zhang W, Dang K, Huai Y, Qian A. (2020). Osteoimmunology: The Regulatory Roles of T Lymphocytes in Osteoporosis. Front Endocrinol (Lausanne), 465-2020.
Publisher | Google Scholor