Case report
Norfloxacin Induced Steven Johnson’s Syndrome: A Case Study
1Ramanbhai Patel College of Pharmacy, Charotar University of Science and Technology (CHARUSAT), CHARUSAT campus, Changa 388421, India.
2 Students, Ramanbhai Patel College of Pharmacy, Charotar University of Science and Technology (CHARUSAT), CHARUSAT campus, Changa 388421, India.
*Corresponding Author: Jalpa Suthar, 1Ramanbhai Patel College of Pharmacy, Charotar University of Science and Technology (CHARUSAT), CHARUSAT campus, Changa 388421, India.
Citation: J. Suthar, P. Nandini, D. Patel. (2024). Norfloxacin Induced Steven Johnson’s Syndrome: A Case Study, Clinical Interventions and Clinical Trials, BioRes Scientia Publishers. 2(1):1-4. DOI: 10.59657/2993-1096.brs.24.011
Copyright: © 2024 Jalpa Suthar, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: December 30, 2023 | Accepted: January 15, 2024 | Published: January 20, 2024
Abstract
Fluoroquinolones have a wide range of activities, which makes them quite popular. Since they can cause T cell-dependent reactions like Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), their benefit-risk profile needs to be carefully examined. Unfavourable drug reactions frequently result in Stevens-Johnsons syndrome (SJS), a rare and sometimes fatal cutaneous reaction. SJS is distinguished in particular by severse epidermal detachment, acute skin blisters, and extensive skin and mucous membrane lesions (including those of the month, nose, oesophagus, anus and genitalia). Drugs were discovered to be a significant factor in the development of SJS in 95% of case report. In the present case, a 59-year-old female patient presented to the hospital with oral lesions, significant oral ulceration, redness, and pain. Her complaint of watery diarrhoea led to the prescription of Norfloxacin. This case study investigated the possibility of a severe hypersensitivity reaction to norfloxacin, which is extremely risky and perhaps lethal.
Keywords: stevens-johnsons syndrome; norfloxacin; fluoroquinolones; adverse drug reactions
Introduction
Antibiotics in the fluoroquinolone class have occasionally been linked to adverse skin responses. Mild rashes to serious skin disorders can result from these reactions. Rash is a typical cutaneous reaction that can occur when using fluoroquinolones. This rash may be moderate and self-limiting, or it may be more serious [1]. Fluoroquinolones, in particular ciprofloxacin, can raise the risk of photosensitivity responses, which make the skin more sensitive to sunlight. This may result in symptoms similar to a sunburn [2]. These skin rashes are severe and potentially fatal. Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) have been linked to fluoroquinolones, including ciprofloxacin, despite these findings being extremely rare [3]. Fixed drug eruptions are localised skin reactions that happen at the same spot with repeated exposure to the drug, and they can occur in some people. A possible cause of these responses is fluoroquinolones [4]. A severe, systemic hypersensitivity reaction called Drug Hypersensitivity Syndrome (DRESS) that can affect the skin. Fluoroquinolone usage has been linked to some incidences [5].
Fluoroquinolones (FQs) are counted among broad-spectrum antimicrobials and are used to treat genitourinary, respiratory, gastrointestinal, skin and soft tissue infections. FQs are generally well tolerated antimicrobials: the discontinuation of treatment due to adverse effect is required in fewer than five percent of consumption. FQs are also associated with serious adverse effect including clostridium difficile infections, prolonged QT interval, tendinitis and tendon rupture [6]. Norfloxacin is a first-generation fluoroquinolone with variable activity gram-positive and gram-negative bacteria. Norfloxacin is indicated in the treatment of acute uncomplicated/complicated chronic recurrent urinary tract infections. Adverse side effect of norfloxacin include prolongation of the QT interval, tendon rupture, hypersensitivity reactions, Stevens Johnson syndrome (SJS- skin hyperpigmentation) and toxic epidermal necrolysis (TEN). Hyperpigmentation refers to patches of skin that became darker than the surrounding area of the skin. It occurs when the skin produces excess melanin, the pigment that gives skin its colour [7].
Case Summary
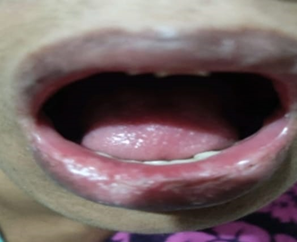
A 59-year-old female presented with a severe lip skin reaction, which was diagnosed as SJS. She had received norfloxacin 400 mg/day for acute gastritis, immediately after finishing the first dose of treatment regimen; she developed cutaneous and mucous lesions on lips typical of mucosal damage and SJS. After a referred to hospital and treatment with oral prednisolone therapy, the female recovered. She had past history of same type of reaction with fluoroquinolone group of antibiotics. Doctor identified the Norfloxacin induced Steven-Johnson syndrome and advised to stop the suspected drug and additionally, advised patients to avoid any fluoroquinolone group of antibiotics in future. Patients had provided consent for the data publishing.
Figure 1: Lips swelling, ulceration erosions, SJS
Discussion
The most common causes of morbidity and mortality are adverse drug reactions (ADRs). Hospital admissions in India are caused by ADRs in the range of 2.9 to 5.6 Percentage [8]. Hospitalisation is necessary for the rare and severe cutaneous ADR known as SJS. FQs have been documented to cause TEN in the past [9]. Sulfa drugs, phenytoin, carbamazepine, lamotrigine, phenobarbital, allopurinol, piroxicam, nevirapine, and diclofenac are the most typical medications with a high risk of causing SJS [10]. Around 40Percentage of drug-induced SJS is attributed to antibiotics [11]. Sulphonamides and penicillins are the antibiotics most frequently linked to drug-induced SJS. Ciprofloxacin (less than 0.01%) and Norfloxacin (0.01Percentage to 0.Percentage) among FQ very infrequently cause drug-induced SJS [12].
Variations in the HLA-B gene lead to abnormal immune responses to the probable medicines that are known to cause SJS. Drug-induced cytotoxic T cells and natural killer cells generate granulysin, which kills skin and mucous membrane cells, as a result of the body's inability to remove reactive metabolites. As a defining trait of SJS, the death of these cells results in skin blistering and peeling. Granulysin content in blister fluid corresponds with the severity of SJS, and CD8+ T lymphocytes have been recognised as crucial blister-forming mediators [13].
In this case, Naranjo’s algorithm [14] was used to determine a plausible reaction due to Norfloxacin. Based on the total score of 8, this SJS was categorized as “probable” reaction to Norfloxacin administration.
Indian guidelines for the treatment of SJS suggested that all suspicious medications be stopped right away. If a patient with SJS has been diagnosed at a primary or secondary healthcare facility, treatment should begin right away, followed by a referral to a tertiary care facility, where the patient will get care in an intensive care unit or an isolated room while a sterile environment is maintained. Additionally, advised to focus on linked co-morbidity while receiving symptomatic treatment IV or IM corticosteroids therapy such as Prednisolone 1–2 mg/kg/day, dexamethasone 8–16 mg/ day and pulse form employing slow intravenous infusion of methylprednisolone 500–1000 mg/day or dexamethasone 100 mg for 3 days should be given within 72 hrs. with total duration of 7–10 days. Assessment of appearing of new lesions, peri-lesional erythema and skin tenderness should be done daily. If steroid is contraindicated in patient i.e. in tuberculosis and severe hyperglycaemia, Cyclosporine 3–5 mg/kg/day for 10–14 days should be used [15].
Table1: Naranjo’s algorithm
| Question | Yes | No | Do not know | Score |
| 1. Are these previous conclusive reports on this reaction? | +1 | 0 | 0 | 1 |
| 2. Did the adverse event appear after the suspected drug was administered? | +2 | -1 | 0 | 2 |
| 3. Did the adverse event improve when the drug was discontinued, or a specific antagonist was administered? | +1 | 0 | 0 | 1 |
| 4. Did the adverse event reappear when the drug was readministered? | +2 | -1 | 0 | 2 |
| 5. Are there alternative causes that could on their own have caused the reaction? | -1 | +2 | 0 | 0 |
| 6. Did the reaction reappear when a placebo was given? | -1 | +1 | 0 | 0 |
| 7. Was the drug detected in blood or other fluids in concentration known to be toxic? | +1 | 0 | 0 | 0 |
| 8. Was the reaction more severe when the dose was increased or less severe when the dose was decreased? | +1 | 0 | 0 | 0 |
| 9. Did the patient have a similar reaction to the same or similar drugs in any previous exposure? | +1 | 0 | 0 | 1 |
| 10. Was the adverse event confirmed by any objective evidence? | +1 | 0 | 0 | 1 |
Total Score 08
Conclusion
The frequency of SJS linked to norfloxacin and ciprofloxacin is quite uncommon. The use of these medications, which have the potential to cause SJS, requires close observation. Doctors should use cautious when prescribing norfloxacin with ciprofloxacin and keep an eye out for such uncommon adverse drug reactions (ADRs).
References
- Ogé LK, Broussard A, Marshall MD. (2019). Acne vulgaris: diagnosis and treatment. American family physician, 100(8):475-484.
Publisher | Google Scholor - Mazumdar G, Shome K. Stevens-Johnson. (2014). Syndrome following use of metronidazole in a dental patient. Indian Journal of Pharmacology, 46(1):121.
Publisher | Google Scholor - RAHMA K. Angka Kejadian Sindrom Stevens Johnson dan Nekrolisis Epidermal Toksik di RSD Dr. Soebandi Jember.
Publisher | Google Scholor - Barbaud A, Collet E, Milpied B, Assier H, Staumont D. et.al. (2013). A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. British Journal of Dermatology, 168(3):555-562.
Publisher | Google Scholor - Mehsas Z, Boubnane I, Sektaoui S, Meziane M, Ismaili N et.al. (2013). DRESS syndrome: A descriptive series of 62 cases. Our Dermatology Online/Nasza Dermatologia Online, 14(3).
Publisher | Google Scholor - Kuula LSM, Viljemaa KM, Backman JT, Blom M. (2019). Fluoroquinolone-related adverse events resulting in health service use and costs: A systematic review. PLoS One, 14(4).
Publisher | Google Scholor - Goldstein EJ. Norfloxacin, (1987). a fluoroquinolone antibacterial agent. Classification, mechanism of action, and in vitro activity. Am J Med, 82(6):3-17.
Publisher | Google Scholor - Jonklaas J, Bianco AC, Bauer AJ, et al. (2014). Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid, 24:1670-1751
Publisher | Google Scholor - Guglielmi R, Grimaldi F, Negro R, et al. (2018). Shift from levothyroxine tablets to liquid formulation at breakfast improves quality of life of hypothyroid patients. Endocr Metab Immune Disord Drug Targets, 18:235-240.
Publisher | Google Scholor - amargo J, Le Heuzey JY, Mabo P. (2015). Narrow therapeutic index drugs: a clinical pharmacological consideration to flecainide. Eur J Clin Pharmacol, 71:549-567
Publisher | Google Scholor - Garber JR, Cobin RH, Gharib H, et al. (2012). Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract, 18:988-1028.
Publisher | Google Scholor - Toward Optimized Practice (TOP) Endocrine Working Group. (2014). Investigation and management of primary thyroid dysfunction clinical practice guideline. Edmonton, AB: Toward Optimized Practice.
Publisher | Google Scholor - Ott J, Promberger R, Kober F, et al. (2011). Hashimoto’s thyroiditis affects symptom load and quality of life unrelated to hypothyroidism: a prospective casecontrol study in women undergoing thyroidectomy for benign goiter. Thyroid, 21:161-167
Publisher | Google Scholor - Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, et al. (1981). A method for estimating the probability of adverse drug reactions. Clin Pharacol Ther, 30:239-245.
Publisher | Google Scholor - Hotha PP, Naidu D, Zachariah NE. (2022). Stevens Johnson Syndrome Associated with Fluoroquinolones: A Case Series. J Pharm Care, 10(4):237-240.
Publisher | Google Scholor