Clinical Trial
Non-Invasive And Neuroimaging Diagnostic Aspects of Acute Transient Hydrocephalus Against Craniocerebral Trauma
1Republican Scientific and Practical Specialized Center for Neurosurgery, Republic of Uzbekistan, Uzbekistan.
2Bukhara branch of the Republican Scientific Center for Emergency Medical Care, Bukhara State Medical Institute, Uzbekistan.
*Corresponding Author: Muminov Murod Djavadovich, Republican Scientific and Practical Specialized Center for Neurosurgery, Republic of Uzbekistan, Uzbekistan.
Citation: Gayrat M. Kariev, Muminov M. Djavadovich. (2023). Non-Invasive And Neuroimaging Diagnostic Aspects of Acute Transient Hydrocephalus Against Craniocerebral Trauma. Journal of BioMed Research and Reports, BRS Publishers. 2(4); DOI: 10.59657/2837-4681.brs.23.030
Copyright: © 2023 Muminov Murod Djavadovich, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: June 23, 2023 | Accepted: July 10, 2023 | Published: July 17, 2023
Abstract
This article presents neuroimaging diagnostic findings such as computed tomography (CT) and magnetic resonance imaging (MRI) in 312 patients with acute transient hydrocephalus traumas. In 119 cases, the characteristic features characterizing the syndrome of intracranial hypertension of a predominantly hydrocephalic origin with development of acute transient hydrocephalus were revealed.
The results of the study presented based on analysis of treated 119 patients with acute transient hydrocephalus of traumatic genesis predetermined the timely revealing of its characteristic features complicating the craniocerebral trauma and permitted to establish direct indications for noninvasive monitoring of intracranial hypertension syndrome of hydrocephalic nature in the acute stage of craniocerebral trauma.
Keywords: craniocerebral trauma; intracranial hypertension; acute hydrocephalus; neuroimaging; computed tomography
Introduction
Traumatic brain injury (TBI) in the structure of morbidity and mortality of the population retains a high frequency, both persistent disability and disability, causing its relevance in modern medicine [6,7,9]. The urgency of the problem of treating patients with TBI is due to difficulties in solving a whole range of tasks, including determining the choice of diagnostic and therapeutic tactics, the need for surgical intervention, their timing and methods of implementation [16,17]. Despite the significant improvement and improvement of the technical equipment of medical institutions, modern achievements in neurosurgery and neuro-resuscitation, the results of treatment of patients with severe forms of TBI, its intracranial complications, remain not completely satisfactory to clinicians [7,9,37,44].
At the present stage of the development of neurosurgery, when choosing an adequate therapeutic and diagnostic tactics, a neurosurgeon, of course, cannot focus only on the data of a clinical and neurological examination. Knowledge of the features of the first clinical and neurological manifestations of the disease, the degree of central nervous system (CNS) dysfunction in acute cerebral insufficiency of traumatic etiology, the course of traumatic brain disease allows us to characterize the general condition of the patient and the degree of preservation of compensatory adaptive mechanisms, which are the key to the correct planning of the scope and sequence of diagnostic measures and interpretation of their results [8,14,36]. As you know, the disease is easier to prevent than to treat. This statement is true for all diseases. But sometimes it happens that it is quite difficult to warn. Because it is not always possible to predict what exactly can provoke a particular patient's condition and aggravate the course of the disease [27,32,41,42,44].
Analysis of literature data, causes of deaths and unsatisfactory results of treatment of patients with TBI of its moderate and severe forms allows us to formulate a number of fundamentally important tactical and technical techniques, without which it is often difficult to obtain satisfactory results of treatment of these nosology’s. Along with this, compliance with certain principles of conducting therapeutic and diagnostic methods in combination with the analysis of clinical and neurological manifestations of the disease allows us to obtain objective criteria for predicting the outcomes of the disease and thereby justify the expediency of using one or another tactic for the management and treatment of patients [10,11,20].
A group of patients with acute transient hydrocephalus, which developed as a complication during organic injuries of traumatic etiology, is difficult to treat and in the absence of timely adequate care, patients die in the first 12-72. The clinic and diagnosis of the acute form of hydrocephalus is rather difficult, since the symptoms of organic damage to the underlying disease, i.e. TBI (both primary and secondary), which level the syndrome of increased "malignant" intracranial pressure of not parenchymal, but hydrocephalic genesis, which causes inadequate treatment of this category of patients [1,2,4,8,22,28], are in the first place.
Acute transient hydrocephalus remains relevant both in neurology and in neurosurgery, which causes close attention to it and the search for its solution. This is due, firstly, largely because adequate conditions for neurosurgical interventions, existing methods of neuromonitoring ICP cannot provided in all patients with acute neurosurgical pathology. Secondly, the point of view existing in the literature about the frequency and pathogenetic mechanisms, the timing of the development of acute transient hydrocephalus, its types and methods of its elimination and prevention is contradictory and rather scarce [3,6,14,22,29].
In this regard, it seems important to address the issue of difficulties encountered in the diagnosis of acute transient hydrocephalus. To a certain extent, this can explain the currently existing high mortality in the group of patients with moderate and severe forms of TBI, as well as the continuing high percentage of both general and postoperative disability in this category of patients [12,18]. In this regard, first it is necessary to understand the causes and timing of the so-called acute transient form of hydrocephalus of traumatic genesis [15].
Aim
The aim of the study was to analyze the characteristic paraclinical and neuroimaging signs in the diagnosis of intracranial hypertension in patients with acute transient hydrocephalus on the background of traumatic brain injury.
Materials & Methods
We reviewed and analyzed objective data such as the results of clinical, neurological and neuroimaging studies of patients with isolated CMT - n=312 cases. All patients treated in Bukhara branch of RSCEMС of Ministry of Health of the Republic of Uzbekistan during the period from 2019 to 2022. Archival and current practice materials used in the work: data from case histories, study protocols, re-examination charts and other medical records of the patients. The work based on the analysis of examination data and treatment outcomes of 119 patients, for craniocerebral trauma with developed acute transient hydrocephalus. The mean age was 38,1±2,4 years (from 18 to 79 years). There were 77(64.71%) male and 42(35.29%) female observations.
The correlation between neurological symptoms and signs of intracranial hypertension in the development of neuroimaging signs of acute hydrocephalus analyzed.
The analysis of the results of treatment of patients with acute transient hydrocephalus of traumatic genesis allowed us to make our own interpretation of the findings. Depending on localization and type of injury to brain structures, the distribution of patients with OTTH against the background of CHT was characterized as follows: supratentorial right-sided - 48(40.3%), men - 32(66.67%), women - 16(33.33%); left-sided - 43(36.13%): men - 24(55.81%), women - 19(44.19%). Subtentorial injury - 28(23.6%): males - 19(67.86%), females - 9(32.14%) (Table 1).
Table 1: Localization and sex data of acute transient hydrocephaly against the background of brain injury.
| Gender | Supratentorial | Sub tentorial | Total | |||||
| Right | Left | |||||||
| Abs | %±0.04 | Abs | %±0.04 | Abs | %±0.04 | Abs | %±0.04 | |
| Men | 32 | 66,67* | 24 | 55,81* | 19 | 67,86* | 75 | 62,68* |
| Females | 16 | 33,33* | 19 | 44,19* | 9 | 32,14* | 44 | 37,32* |
| Total | 48 | 40,3* | 43 | 36,1* | 28 | 23,6* | 119 | 100,0^ |
| Note * | - Differences between male and female data are significant (P less than 0.01) ^ - the differences between Group 1 and Group 2 are significant (P less than 0.05) | |||||||
The distribution of patients according to the nature of the traumatic substrate was as follows. Traumatic epidural hematoma was detected in - 31(26,05%), subdural hematoma - 24(20,17%), contusion (mild, moderate, severe), intracerebral hematoma with foci of crush - 64(53,78%). (Table 2).
Table 2: Localization and nature of injury in acute transient hydrocephaly caused by brain injury.
| Localization | Nature of injury | Total | ||||||||
| Epidural hematoma | Subdural hematoma | Brain contusion | ||||||||
| Abs | %±0,04 | Abs | %±0,04 | Abs | %±0,04 | Abs | %±0,04 | |||
| Supratentorial | Right | 14 | 11,76* | 12 | 10,08* | 22 | 18,49* | 48 | 40,3* | |
| Left | 11 | 9,24* | 9 | 7,56* | 23 | 19,33* | 43 | 36,1* | ||
| Sub tentorial | 6 | 5,05* | 3 | 2,53* | 19 | 15,96* | 28 | 23,6* | ||
| Total | 31 | 26,05 | 24 | 20,17 | 64 | 53,78 | 119 | 100,0^ | ||
| Note * | - Differences between male and female data are significant (Pless than 0.01) ^ - the differences between Group 1 and Group 2 are significant (P less than 0.05) | |||||||||
The stage of the disease was classified according to the grading proposed by Prof. A.L. Polenov Russian Neurosurgical Institute (1984), which included compensated (I), sub compensated (II), decompensated: moderate (III) and grossly decompensated (IV). The paraclinical methods of investigation in patients with OTTH caused by traumatic brain injury included standard general and biochemical blood tests, ophthalmological, otoneurologic tests. The instrumental methods of investigation were applied: cardiography of the skull in standard projections - n=119(100%) patients, electroencephalography - n=64(53,78%), Echo-ES - n=119(100%). Computed tomography data of 119(100%) and magnetic resonance imaging of n=29(24,37%) patients and control MSCT-images in the dynamics of treatment of 119(100%) patients were studied.
The adjacent specialists examined patients in dynamics of observation and treatment for brain injury complicated by acute transient hydrocephaly: ophthalmologist and otoneurologic. Computed tomography and MRI are imaging techniques of the brain, intracranial spaces as well as bones and soft tissues. CT was performed on Multiplied computed tomography (CT), Revolution-EVO (64), Japan and on Siemens "Somatom AR TX" (Germany) with a scanning layer width of 2 to 10 mm, current strength of 50 to 70 mA, with tube current voltage of 110 to 130 kV and apparatus at a maximum Gentry angle (GT) of ±28°. Structural organic lesions of the brain (nature, volume, localization of the pathological substrate, HFH), distribution, and degree of ventricular dislocation were taken into account during the examination. The density of prepathological brain tissue and its Hounsfield density were determined. Patients with suspected changes from the skull bone sheath subjected to a CT scan in bone mode to detect traumatic changes from the skull base bones.
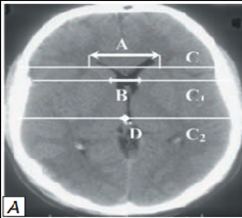
CT examination - 119(100%) and MRI data from 29(24.37%) patients allowed us to identify characteristic changes in the cerebrospinal circulation system, which was essential in terms of non-invasive neuroimaging monitoring of intracranial prosthion in the development of acute transient hydrocephaly. To objectify the pathological states of ventricular system, its position, magnitude, and degree of displacement were studied according to CT of the brain with calculation of VCC, diameter of retroorbital part of optic nerves (dON), and cerebra-ventricular Evans Index (the norm 20.0±1.85). At detection of acute hydrocephalus, we calculated ventriculi-cranial coefficients (VCC-1, VCC-2, VCC-3, VCC-4 and VCC - ventricular bodies) according to the accepted method and compared the values with age norms.VCC-1 is the ratio of the index between the anterior horns of the lateral ventricles to the value between the cranial vault bones. Normal value of VCC-1 according to S.B. Vavilov (1986): 24.0-26.3% in the age group under 60 years old, 28.2-29.4% in the age group over 60 years old. VCC-2 is the ratio of the anterior horn bodies between the heads of the caudate nuclei to those between the frontal lobes. The VCC-2 (according to V.N. Kornienko, 1987) is 36 years- 16%; 36 to 45 years-17%; 46 to 55 years - 18%; 56 to 65 years-19%; 66 to 75 years-20%; over 76 years-21%.
VCC-3 calculated as the ratio of the size of the third ventricle to the distance between the bones in the cranial vault. In terms of age, VCC-3 was characterized as follows: at 30 years of age, 2.7%; from 31 to 40 years, 2.9%; from 41 to 60 years, 3.5%; from 61 to 70 years, 3.9%; over 70 years, 4.3% (according to N.V. Vereshchagin, 1986). VCC-4 - the ratio of the size of the IV ventricle to the diameter of the posterior cranial fossa was determined. Normal values of VCC-4 are constant in any age group. According to N.V. Vereshchagin (1986), the normal rate of VCC-4 is 11.3-13%.
The VCC-body determined by the ratio of the size of the removed portions of the cranial vault and the edges of the bodies of the lateral ventricles to the size between the inner plates of the skull bones. Normal values for VKC-bodies according to N.V. Vereshchagin (1986) were 18.4-26.0%. We used the calculation of normal values of VCC according to the methodology accepted in Scientific Research Institute of Emergency Care named after N. Sklifosovsky. V. Puras, E.V. Grigoryeva, Lectures in Neurosurgery, No. 2, 2014).
In our work, we adhered to the calculation of the following coefficients:
- VCC of ventricular bodies;
- Enlargement of the 3rd ventricle;
- Ventriculi-cranial coefficient increase - 2 over 9%;
- Retro-orbital optic nerve sheath size;
- dON to 3rd ventricle size ratio - HgS.
VCC-1 - (VCC-1=A/Cx100%) (Evans Index) up to age 60 years - 24.0-26.3%, in the age group over 60 years - 28.2-29.4%
VCC-2 - (VCC-2=B/C1x100%): at the age of 30 - 16%, 31-40 years - 17%; 41-60 years - 18%, 61-70 years - 19%, 71-80 years - 20%, over 80 years - 21%
BCC-3g - (BCC-3g = D/C2 x 100%) in patients under 30 years of age 2.7%, 31-40 years - 2.9%, 41-60 years - 3.5%, 61-70 years - 3.9%; over 70 years - 4.3%
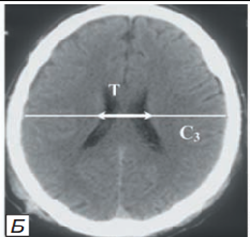
VCC bodies - (VCC-body=T/C3x100%) VCC body values are 18.4-26.0%
VCC4g - (VCC-4g=F/Gx100%), normal value is 11.3-13%
The diagnosis of acute transient hydrocephalus against the background of traumatic brain injury based on clinical, neurological, neuroimaging data with the presence or absence of signs of brain structure dislocation at traumatic intracranial haematomas, but also the presence of progressive intracranial hypertension. The treatment and management of patients with traumatic intracranial hematomas is currently determined not only and not so much by establishing the fact of intracranial hypertension, as by the clinical picture of a progressive, often "malignant" intracranial hypertension acute hydrocephalus syndrome.
Results and Discussion
In all cases, we noted a sign of progressive "malignant" intracranial hypertension, which manifested by increased Evans’s index and enlarged width of subarachnoid slits and sulci. Depending on the time of hospitalization, the patients distributed as follows: 0-3 days - 91(76.47%), 4-7 days - 24(20.17%), 8-10 days - 4(3.36%). Road traffic accidents resulted in 52.1% injuries, street injuries (including criminal injuries) in 21.1%, domestic injuries in 11.9%, and falls from height in 3.36% of the victims. (Table 3).
Table 3: Distribution of patients with acute transient hydrocephalus of traumatic genesis by period of hospitalization and type of injury.
| Type of trauma | Time of hospitalization from the moment of trauma | Total | ||||||
| 0-3 days | 4-7 days | 8-10 days | ||||||
| abs | %±0,04 | abs | %±0,04 | abs | %±0,04 | abs | %±0,04 | |
| Street | 13 | 10,92 | 14 | 11,76 | 3 | 2,52 | 30 | 21,1 |
| Household | 12 | 10,08 | 2 | 1,68 | - | - | 14 | 11,9 |
| Transport | 62 | 52,1 | 6 | 5,04 | - | - | 68 | 62,1 |
| High-rise | 4 | 3,36 | 2 | 1,68 | 1 | 0,84 | 7 | 4,9 |
| total | 91 | 76,47 | 24 | 20,17 | 4 | 3,36 | 119 | 100,0 |
Chronographic examination was performed in all 119 (100%) patients and was regarded by us as a mandatory component of the neurosurgical diagnostic complex. Thus, fractures of the bone sheath were identified in 79(66,38%): cranial vault (including depressed fractures - n=18) 65(82.28%); skull base fractures (including facial skeleton - n=11) were noted in 14(17.72%) (Table 4).
Table 4: Radiological findings in patients with acute transient hydrocephalus on the background of brain injury.
| Chronographic changes | in EG (n=31) | in SG (n=24) | In brain contusion (n=64) | Total | ||||
| abs | %±0,04 | abs | %±0,04 | abs | %±0,04 | abs | %±0,04 | |
| skull fracture | 15 | 48,39** | 13 | 54,17** | 37 | 57,81** | 65 | 54,62^ |
| fracture of skull base | 4 | 12,9 | 3 | 12,5 | 7 | 10,94* | 14 | 16,9^ |
| depressed fracture | 9 | 29,03 | 2 | 8,33 | 6 | 9,38 | 18 | 12,68^ |
| facial fracture | 3 | 9,68 | 1 | 4,17* | 7 | 10,94 | 11 | 14,79^ |
| Total | 19 | 61,29%* | 16 | 66,67%* | 44 | 68,75%* | 79 | 66,38 |
| Note: | * - differences between male and female data are significant (** - P less than 0.01) ^ - differences between groups are significant (^ - P less than 0.05) | |||||||
Targeted X-ray examination of the bones of the facial skull made in 21 (17.64%) victims, 11 patients had fractures of the facial skeleton bones (nasal bones, malar bone and lower jaw). Neuroophthalmological examination performed in all (n=119) patients with acute transient hydrocephalus caused by brain injury and included examination of the functional state of the visual organ: visual acuity and field width, fundus examination by indirect ophthalmoscopy, crania-cerebral nervous function status was determined. The changes on the ocular fundus were evaluated according to 4 stages of optic disc stasis. Stagnant optic discs reflecting the severity of hydrocephalic-hypertensive syndrome predominated in the group with brine contusion and fracture foci (n=64) - 59(92.18%) of observations (p less than 0.05).
A sharp decrease in visual acuity detected in 18(12.68%) patients. (p less than 0,05). Increased ICP mainly in the ventricular system led to the development of congestive papillae of the optic nerve disc. Pupillometry in patients revealed a persistent increase of intraocular pressure with a change of pupil size and revealed anisocoria in 102(85,71%) while objective examination revealed anisocoria in 72(60,5%) cases only.
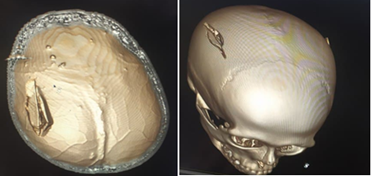
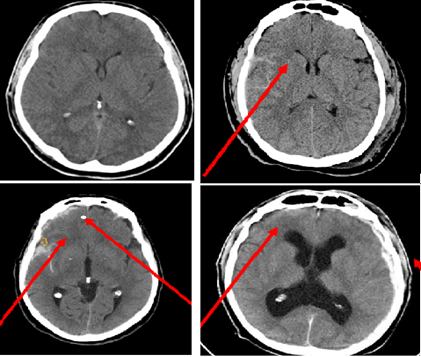
Figure 1: Cranial vault fracture: depressed fracture of the parietal bone on the right.
Oto-neurological examination of 109(91.6%) patients with acute transient hydrocephalus against the background of brain injury revealed vestibular (optokinetic and caloric nystagmus, vertigo), reflecting presence or absence of hypertensive components on supra- or sub tentorial level and their lateralization. Oto-neurological examination consisted of tonal and colloquial speech tests, and examination of the tympanic membrane. In all observations, the presence of a swollen eardrum identified as one of the criteria for elevated ICP. The study of vestibular function (which significantly hampered by the general condition of the patients!) consisted in determining the amplitude and nature of spontaneous, caloric and optokinetic nystagmus.
Echo-encephalectomy performed in all 119 patients with acute transient hydrocephalus of traumatic genesis using "Echo-12" and "Angiodin-Echo/M" devices. A common technique of head probing used to determine the size, width and nature of the medial structures (M-echo) of the brain. The presence of intracranial hypertension was manifested by splitting and widening of the medial complex, the appearance of additional (multiple) echo signals, and an increase in the cerebral cloak index. In recent years, CT and MRI examinations have been widely used in the diagnosis of acute neurosurgical pathology. However, the accumulated experience of using CT scanning demonstrated that even modern computer equipment having higher resolution may not always reflect true lesions of brain structures in brain injury and frequently (up to 41.0%) cannot visualize typical signs of cerebrospinal fluid secretion, liquor circulation, and liquor adsorption disorders.
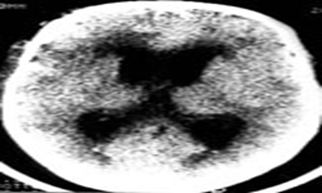
In all observations (n=119) we noted the sign of progressive "malignant" intracranial hypertension manifested by increased Evans’s index, decreased VCC-t and narrowed width of subarachnoid slits and sulci. The patterns of changes in the shape of brain matter structures, namely the ventricular system, brain base cisterns, and other subchondral spaces, depending on the nature and severity of structural lesions, sufficiently assessed by CT data. In hydrocephalus syndrome with increased intraventricular pressure, characteristic changes detected in the lower horn of the lateral ventricles and their width exceeded 3 mm (Figure 2).
Figure 2: CT signs of acute internal hydrocephalus with dilated inferior horns of the lateral ventricles.
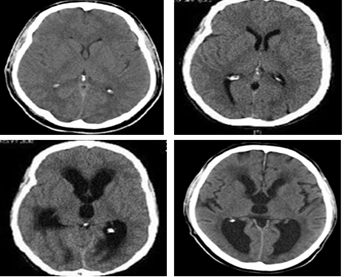
Generally, periventricular edema detected in the anterior horns of the lateral ventricles. (Figure 3).
Figure 3: CT signs of acute internal hydrocephalus with increased size of the anterior and posterior horns of the lateral ventricles.
Changes in ventricle III according to neuroimaging data characterized by "swelling" and balloon-shaped deformation of its contours. Changes in the width of the third ventricle are considered to be the most reliable sign of hydrocephalus, normally not exceeding 8 mm in width and the nature of the lateral ventricular walls should be assessed (Figure 4).
Figure 4: Parameters of changes in the size of the 3rd ventricle according to neuroimaging data in the development of acute transient hydrocephalus.
Changes in the width of ventricle III is considered the most reliable sign of hydrocephalus, normally not exceeding 8 mm in width and the nature of the lateral ventricular walls should be assessed [9]. The combination of conducting and calculating the indices of brain structures in CCI based on CT monitoring allowed predetermining the risk of developing acute transient hydrocephalus with the syndrome of "malignant" progressive intracranial hypertension. This fact allowed in further tactics of treatment and follow-up of patients, to conduct active either non-invasive or invasive monitoring of ICH with installation of intrathecal (ventricular, subdural) sensor measuring ICP.
Measurement of the size of the 3rd ventricle, its enlargement and expansion, being a predictor of the development of acute hydrocephalus, which allowed us to apply its indicators when using the formula for calculating the hypertensive and hydrocephalus syndrome:
- HHS - hypertension-hydrocephalus syndrome
- dP - pupil diameter (pupillo) (N=2-9mm)
dNOs - mean optic nerve size (difference between exit from cranial cavity and retinal area - extraocular-extracranial part (orbital or retrobulbar) deep physiological escavation (4.0-4.5 mm, 3 shells). pVIII ventricular floor size 
We measured dPH 2 mm from the dorsal contour of the eyeball (d1) and 2 mm from the cranial cavity (d2). The mean value of dON width (ds) calculated according to the formula: 
ICP in the control group (healthy) was 1.0±0.03

The gradient of the correlation between dsS size and 3rd ventricle width exceeded 1.0, we considered a hypertensive syndrome (HtS) of parenchymatous genesis due to cerebral edema.

When the correlation coefficient of dsS and 3rd ventricle width did not exceed 1.0, we considered HtS as hydrocephalic syndrome (HgS-hyper production, hypo resorption of cerebrospinal fluid, occlusion of the cerebrospinal pathways).

As described earlier, non-invasive CT-monitoring of HgS condition allowed us to predetermine the development of OTFH and, if it develops, to carry out adequate therapeutic and diagnostic tactics in the future. Primary monitoring of patients with traumatic epidural and subdural hematomas performed from the moment of their admission to the hospital. The findings of primary CT monitoring (0-3 days after injury) in patients with acute transient hydrocephaly for acute traumatic envelope hematomas (EH, SH) presented in Table 8.
Table 8: Indicators of primary CT-monitoring of ICH in envelope hematomas.
| Predictor of ICP | against EH (n=31) | against SH (n=24) |
| VCC-t (18,4-26,0) | 16,7±0,28 | 15,9±0,26 |
| Evans CVI (<24> | 32,0±0,19 | 31,2±0,21 |
| dON (4,0мм) | 5,1±0,13 | 5,3±0,16 |
| V3 (4,5мм) | 7,2±0,17 | 7,6±0,14 |
| HgS | 0,71±0,08 | 0,7±0,09 |
Thus, the cerebral ventricular body ratio (VCC-t) in acute transient hydrocephaly against acute traumatic EH averaged 16.7±0.28, whereas in SH it was 15.9±0.26. Evans' CVI measurement against acute traumatic envelope hematomas resulting in acute transient hydrocephaly was 32.0±0.19 in EH and 31.2±0.21 in SH respectively. We obtained an Hg S of 0.71±0.08 in EH and 0.7±0.09 in SH. The findings of primary CT-monitoring (0-3 days from injury) in OTFH patients with cerebral contusions, traumatic intracerebral hematomas (TICH) and crush injuries are presented in Table 9.
Table 9: Primary CT-monitoring indices of ICH in contusion brain injury.
| Predictor of ICP | against brain injury (n=64) |
| VCC-t (18,4-26,0) | 17,3±0,21 |
| Evans CVI (<24> | 31,1±0,14 |
| dON (4,0мм) | 5,8±0,14 |
| V3 (4,5мм) | 7,0±0,17 |
| HgS | 0,83±0,07 |
Thus, the cerebral ventricular body coefficient (VCC-t) in acute transient hydrocephaly against the background of brain injury averaged 17.3±0.21. The Evans CVI measurement on the background of contusion and cerebral crush with intracerebral hematomas with the development of OTFH was 31.1±0.14, respectively. HgS was 0.83±0.07 in the diagnosis of malignant hydrocephalus syndrome in contusion brain injury and averaged 0.83±0.07.
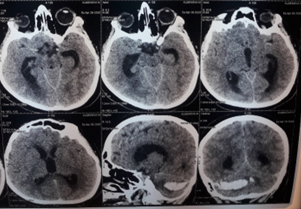
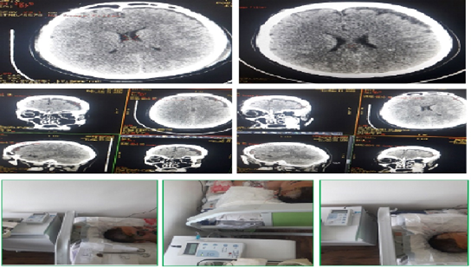
Figure 5: Patient Sc.D., born in 1990. Diagnosis: Closed craniocerebral injury. Brain injury of moderate severity of the middle degree of the frontal area on the right. Acute traumatic subdural hematoma of the left hemisphere, complicated by acute transient hydrocephalus (before surgery): a) at the time of admission (1 hour); b) the first day (24 hours); c) on the 3rd day (72 hours); d) on the 5th-7th day.
Figure 6: Patient Sc.D., born in 1990. Diagnosis: Closed craniocerebral injury. Brain injury of moderate severity of the middle degree of the frontal area on the right. Acute traumatic subdural hematoma of the left hemisphere, complicated by acute transient hydrocephalus (before and after the operation of cranial trefenation with removal of hematoma and establishment of subdural drainage (invasive monitoring of ICP)).
The diagnosis of acute transient hydrocephalus with intracranial hypertension, in contrast to primary structural brain lesions, based primarily on both clinical data and immediately after confirmation of its development by neuroimaging. The primary causes of hydrocephalus with intracranial hypertension secondary to traumatic brain injury, such as epi-, subdural and intracerebral hematomas, brain contusions and crush injuries discussed.
Conclusion
Dynamic noninvasive neuroimaging monitoring to determine the presence or absence of a hydrocephalic component of ICH, with comparison to noninvasive paraclinical ICP monitoring methods, should performed in patients with acute transient hydrocephaly against the background of a traumatic brain injury.
On the basis of the findings confirming the development of acute transient hydrocephalus, the indication for invasive method of ICH monitoring with placement of external ventricular or subdural drainage is predetermined.
Adequate neuroimaging diagnostics with calculation of coefficients of intracranial hypertension is the key to success in treatment of acute cerebral insufficiency developed in a traumatic brain injury.
References
- Gafurov B.G. (2011). Intracranial hypertension syndrome. Methodical guidelines, 34 с.
Publisher | Google Scholor - Efimtsev A.Yu. (2013). Visualization of cerebrospinal fluid movement using MPT - TIME-SLIP technique. Nevsky Radiologichesky Forum, 256с.
Publisher | Google Scholor - Zamyshlyaev P.S., Plotnikova N.A., Kemaykin S.P. (2015). Hydrocephalic syndrome. Pathomorphology, prevalence in the Republic of Mordovia. Medico-pharmaceutical journal Pulse.
Publisher | Google Scholor - Kariev M.H. (2000). State of neurosurgical service in the Republic of Uzbekistan. Jurn. of theoretical and clinical medicine, С.20-25.
Publisher | Google Scholor - Kasumov R.D., Klimenko N.B., Mukhtarov R.I. (1995). Diagnosis and treatment of hypertension-dislocation syndrome in patients with severe craniocerebral injuries. X European Congress of Neurochir.: Theses abstracts – Berlin, 32.
Publisher | Google Scholor - A.N.Konovalov (2001). Clinical Manual for Craniocerebral Trauma. Т. 2. ed. by. Moscow: Antidor.
Publisher | Google Scholor - Kornienko V.N. (1998). Computer tomography: In book Clinical guidelines on craniocerebral trauma, ed. by A.N. Konovalov, L.B. Likhterman, A.A. Potapov. M.: Antidor, 472-494.
Publisher | Google Scholor - Krylov V.V., Talypov A.E., Puras Y.V. (2007). Intracranial pressure in brain injuries. Neurosurgery, 4:12-19.
Publisher | Google Scholor - Latyshev A.Y., Kravchuk A.D., Likhterman L.B. et al. (2018). Modern diagnosis and treatment of posttraumatic hydrocephalus. Introduction of neurosurgery named after N.N. Burdenko. N.N. Burdenko. 2:81-7.
Publisher | Google Scholor - Likhterman L.B. (2014). Craniocerebral trauma. Diagnosis and treatment. M.: GEOTAR-Media.
Publisher | Google Scholor - Mamadaliev A.M. (1998). Prediction of outcomes of craniocerebral trauma in the acute period: Autoref. dissertation. D. in medical sciences. M.: Research Institute of Neurochir. named Burdenko N.N., 41.
Publisher | Google Scholor - Makhkamov K.E., Yunusov R.S., Khusankhojaev J.U. (2011). The place and role of computed tomography in the diagnosis and selection of management tactics of patients with severe craniocerebral trauma. Vestnik of Emergency Medicine, 32-35.
Publisher | Google Scholor - Mirzabaev M.J., Kariev G.M., Baratov B.I. (2011). Diagnosis and treatment tactics of severe craniocerebral trauma in the aspect of intracranial hypertension. 199.
Publisher | Google Scholor - Mirsadykov D.A. (2004). Diagnosis and treatment of normotensive hydrocephalus. D. thesis of Doctor of Medicine. -St. Petersburg, 387.
Publisher | Google Scholor - Mukhametzhanov H. (2002). Pathology of the spinal cord system in craniocerebral trauma. Author's abstract of dissertation. doc.med -Moscow, 28.
Publisher | Google Scholor - Semyonov A.V., Sorokovikov V.A. (2015). Non-invasive measurement of intracranial pressure in clinical practice (review of literature) Bulletin of All-Russian Scientific Center of the Russian Academy of Medical Sciences, 3:103
Publisher | Google Scholor - Turkin A.M., Oshorov A.V., Pogosbekyan E.L., Smirnov A.S., Dmitrieva A.S. (2017). Correlation of intracranial pressure and optic nerve sheath diameter according to computed tomography in severe craniocerebral trauma. Journal. Voprosy neurosurg., 81-88.
Publisher | Google Scholor - Abstracts from hydrocephalus (2018). The tenth meeting of the International Society for Hydrocephalus and Cerebrospinal Fluid Disorders. Fluids Barriers CNS, 15:35.
Publisher | Google Scholor - Balestreri M., Czosnyka M., Hutchinson P., et al. (2006). Impact of intracranial pressure and cerebral perfusion pressure on severe disability and mortality after head injury. Neurocrit. Care, 4:8-13.
Publisher | Google Scholor - Bundgaard H., Cold GE. (2000). Studies of regional subdural pressure gradients during craniotomy. Br. J. Neurosurg., 14(3):229-234.
Publisher | Google Scholor - Geeraerts T, Launey Y, Martin L, Pottecher J, Vigué B. (2007). Ultrasonography of the optic nerve sheath may be useful for detecting raised intracranial pressure after severe brain injury. Intensive Care Med, 33:1704-1711.
Publisher | Google Scholor - Hansen HC, Helmke K. (1997). Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: ultrasound findings during intrathecal infusion tests. J.Neurosurg., 87:34-40.
Publisher | Google Scholor - Kalantari H, Jaiswal R, Bruck I, Matari H, Ghobadi F. et al. (2013). Correlation of optic nerve sheath diameter measurements by computed tomography and magnetic resonance imaging. Am J. Emerg Med., 31:1595-1597.
Publisher | Google Scholor - Li, Guichen MD; Wang, Guangming MD; Luan, Tengfei MD; Hou, Kun MD; Yu, Jinlu. (2020) Acute hydrocephalus secondary to traumatic perimesencephalic pneumocephalus. Medicine, 99(5).
Publisher | Google Scholor - Rajajee V., Vanaman M., Fletcher J.J., Jacobs T.L. (2011). Optic nerve ultrasound for the detection of raised intracranial pressure. Neurocrit Care. 15:506-515.
Publisher | Google Scholor - Sekhon M.S., Griesdale D.E., Robba S., McGlashan N., Needham E. et al. Optic nerve sheath diameter on computed tomography is correlated with simultaneously measured intracranial pressure in patients with severe traumatic brain injury. Intensive Care Med. 40:1267-1274.
Publisher | Google Scholor - Tayal VS, Neulander M, Norton HJ, Foster T, Saunders T, Blaivas M. (2007). Emergency department sonographic measurement of optic nerve sheath diameter to detect findings of increased intracranial pressure in adult head injury patients. Ann Emerg Med., 49:508-514
Publisher | Google Scholor - Vos P.E. et al. (2002). Traumatic Brain Injury. Eur. J. Neurol., 9(3):207-219.
Publisher | Google Scholor - Zakharova N, Kornienko V, Potapov A, Pronin I. (2014). Neuroimaging traumatic brain injury. Neuroimaging of Traumatic Brain Injury, 1-159.
Publisher | Google Scholor