Case Report
Monitoring Protocol for Buprenorphine Treatment for Opioid Use Disorder Patients
1Department of Biomedical Informatics, State University of New York (SUNY) at Buffalo, Buffalo, NY, United States.
2Arnold School of Public Health University of South Carolina 915 Greene Street, United States.
*Corresponding Author: Hiroko Furo, Kirsty Smith, Department of Biomedical Informatics, State University of New York (SUNY) at Buffalo, Buffalo, NY, United States.
Citation: Furo H., Smith K. (2024). Monitoring Protocol for Buprenorphine Treatment for Opioid Use Disorder Patients. Journal of BioMed Research and Reports, BioRes Scientia Publishers. 5(2):1-6. DOI: 10.59657/2837-4681.brs.24.097
Copyright: © 2024 Hiroko Furo, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: June 28, 2024 | Accepted: July 18, 2024 | Published: August 01, 2024
Abstract
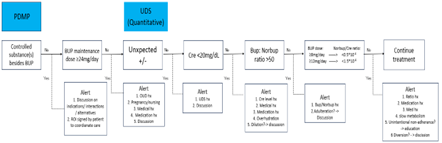
This paper proposes an algorithm for monitoring buprenorphine treatment for opioid use disorder (OUD). This algorithm integrates Prescription Drug Monitoring Program (PDMP) reports and Urine Drug Screening (UDS) test results. PDMPs can reveal a patient’s-controlled substance prescription history and buprenorphine treatment progress, while UDS results may demonstrate buprenorphine treatment adherence and illicit substance use history. The algorithm focuses on the following concerning features: 1) Controlled substance prescriptions besides buprenorphine listed in PDMPs, 2) Maintenance buprenorphine dose is >24mg/day, 3) Unexpected UDS results; Positive substances are detected besides buprenorphine and norbuprenorphine; buprenorphine and/or norbuprenorphine are not detected, 4) Urine creatine level is <20mg/dL, 5) The ratio between buprenorphine and norbuprenorphine is >50 in UDS, and 6) For patients whose daily buprenorphine does is ≥8mg, the ratio of norbuprenorphine to creatine is <0.5; for those with daily buprenorphine dose ≥12mg, the ratio is <1.5. The algorithm assists prescribers in paying close attention to each feature and suggests appropriate interventions for each step, which focuses on patient-centered interventions such as education on buprenorphine intake and diversion preventions. The algorithm can be incorporated into the Electronic Medical Record (EMR) as the clinical decision support system, which would result in potential enhancements for better monitoring with the interoperability of EMRs and UDS laboratory systems. In particular, this would provide prescribers with easy access to a chronological display of norbuprenorphine to creating ratios in relation to buprenorphine dose. The paper concludes by emphasizing the algorithm’s role in improving monitoring accuracy without punitive intentions, fostering patient-centered care.
Keywords: monitoring protocol; buprenorphine treatment; opioid use; disorder patients
Introduction
In the U.S., the number of opioid overdose deaths, especially due to illicit opioid use, has been increasing drastically. The National Institute of Drug Abuse (NIDA) reported 80,411 opioid overdose deaths in 2021 [1]. The U.S. government and its agencies have implemented various measures to control this epidemic such as naloxone distribution programs, funding for research, and training for health care professionals [1]. State governments also joined the effort. One of the ways they have tried to combat this epidemic is through Prescription Drug Monitoring Programs (PDMPs), which allow prescribers to access information on controlled substance prescriptions. In 2023, Missouri activated its PDMP, which means that all states now have a state-wide functional PDMP [1]. There are still some concerns, such as varying information within each state PDMP, ineffective and insufficient utilization in clinical practice, limited interstate sharing, the lag time in updating data, and no listing of in-clinic dispensed medications such as methadone, but there is at least a functional PDMP in all 50 states at present [2,3].
In addition, the federal “X waiver” requirement was removed in 2023, so prescribers can prescribe buprenorphine for OUD patients without obtaining approval from Substance Abuse and Mental Health Services (SAMHSA). This legislation change allows any qualified prescribers to prescribe buprenorphine with only eight hours of training [1]. This waiver elimination made it less onerous for prescribers to prescribe buprenorphine with the goal that more buprenorphine would be prescribed to help OUD patients, which would then expectantly lower opioid overdose deaths. Due to this federal regulation change, more prescribers are able to prescribe buprenorphine with less effort, which has led to a higher need for a simple monitoring protocol for buprenorphine treatment. Therefore, this study proposes an algorithm that can be incorporated into EMR, so that prescribers can monitor their OUD patients’ treatment progress who are on buprenorphine.
PDMP
Many states mandate that prescribers must review their state’s PDMP before prescribing any controlled substances [1]. Checking the PDMP is particularly important in addiction medicine because then prescribers know if their patient is receiving any other controlled substance(s) from another prescriber. These controlled substances, such as other opioid agonists and benzodiazepines, will interact with buprenorphine, resulting in serious adverse effects [2]. For patients receiving these controlled substances, the prescriber can discuss with the patients the indication of the medications, interactions between these medications and buprenorphine, and possible alternatives to non-controlled substance medications. It is also important to have the patient sign a release of information (ROI), so that their prescribers can coordinate care.
In addition to the information on controlled substance prescription, reviewing the PDMP will give the prescriber ample information on the patient’s buprenorphine prescription history and OUD treatment progress. Prescribers can know when the patient started buprenorphine treatment, if the patient’s dose and prescription frequency have been consistent or fluctuating, and if the patient has changed their prescribers frequently. This information would give the prescriber some idea as to whether the patient's treatment is stable or not, e.g., early remission (> 3 months and less than 12 months), sustained remission, etc. [2]. In addition, if the patient is in the maintenance phase, the prescriber needs to pay attention to the latest dose because it would likely be the one the prescriber continues with on that visit unless there is any reason to change it.
The prescriber can check to see if the patient is in the maintenance phase and if so, the buprenorphine dose should be ≤ 24mg/day in most cases. SAMHSA recommends up to 16mg of buprenorphine during the maintenance phase [2, 3]. Greenwald (2014) looked at brain PET scans of patients with different buprenorphine doses and found that approximately 79-95% of mu-opioid receptors were occupied with 16 mg of buprenorphine, which was similar to those who were on >24 to 32 mg (up to 95%), indicating that opioid receptor occupancy is not much different between 16mg/day and 32mg/day [2]. Furo, et al. (2022 and 2023) looked at the buprenorphine dose distribution among the OUD patients who were residing in a monitored environment and found that the average buprenorphine daily dose was approximately 11mg (range from 2mg – 20mg) [2, 3]. Another study examined the risk factors of relapse and found that there was an association between higher doses of buprenorphine prescription and a higher risk of relapse [2]. Patients with extra buprenorphine might be more tempted to divert their buprenorphine. Also, adverse effects of buprenorphine at higher doses need to be taken into consideration as a quality-of-life issue for patients [2]. For all these reasons, prescribers should pay closer attention to patients on more than 24mg/day of buprenorphine.
However, there might be cases in which over 24 mg of buprenorphine is necessary. Temporary or acute cases include: 1) pregnant women, especially in the third trimester, 2) women who are nursing due to their higher metabolism, [4,5,6]. 3) patients who need post-operative buprenorphine re-titration [2], and 4) patients who suffer from precipitated withdrawal [2]. Chronic cases include: 1) fast metabolizers, 2) patients with medical conditions that would alter buprenorphine metabolism [2, 6, 7]. and 4) patients who are on CYP450 3A4 inducing medications [8, 9]. Due to the prevalence of recent fentanyl use, patients with certain OUD history might have developed a higher tolerance to opioids, so they may need a higher dose of buprenorphine [1]. Thus, if a patient asks for >24 mg/day of buprenorphine, the prescriber should have a discussion with them as to why they request a higher dose of buprenorphine.
UDS
Urine drug screening (UDS) tests are another important tool to monitor patients’ treatment progress. There are two types of UDS tests, qualitative and quantitative. This paper focuses on quantitative UDS result interpretation with the levels of buprenorphine and norbuprenorphine, a metabolite of buprenorphine, as well as creatinine.
Quantitative UDS tests have both lower and upper cut-off levels. The cut-off levels vary depending on the laboratory and the substance that is being tested. If the substance is diluted to a level lower than the lower cut-off level of each test, the result will come back as undetectable (non-positive). In addition to cut-off levels, the detection window of each substance would affect the test results. For example, the detection window of buprenorphine and norbuprenorphine is 2 to 4 days but even longer for those who take buprenorphine chronically [3]. Furthermore, the timing of the substance intake to the urine collection affects the test results. Kronstrand, et al. (2008) found that the ratio between buprenorphine and norbuprenorphine will reverse after 7 hours of a single buprenorphine dosing, i.e., buprenorphine level would be higher than norbuprenorphine shortly after the intake, but norbuprenorphine levels will surpass that of buprenorphine 7 hours after the intake [4]. Thus, many factors can affect buprenorphine and norbuprenorphine levels in urine.
Keeping this in mind, the first thing to check on quantitative UDS results is to see if they match the prescription, especially with the PDMP information. If patients are adherent to their buprenorphine treatment, their UDS results should be positive with buprenorphine and norbuprenorphine without any illicit substances. If this is not the case, the prescriber needs to talk with the patient to find out the reasons for the discrepancy. They might also need to educate the patient on the risks of combining buprenorphine with other illicit substances [11]. If previous UDS results are available, prescribers can see the results chronologically to understand their substance use history.
Urine samples can be diluted by adding water. Urine creatinine levels should be reviewed for this kind of adulteration. If creatinine concentration is <20mg> 2mg/dL are still physiologically possible for various reasons: 1) patients’ age and sex (elderly and female tend to have lower creatinine levels), 2) certain medications such as diuretics, 3) illnesses, such as kidney disease and polydipsia due to diabetes insipidus, and 4) excessive hydration, possibly caused by the effort to overcome urinary retention, a rare adverse effect by buprenorphine intake [14]. So, when creatine is less than 20mg/dL, prescribers should discuss the results with the patient and specifically review their demographic information, medical history, current medication(s), and so forth before considering the possibility of dilution.
Then, the prescribers can check the ratio between buprenorphine and norbuprenorphine. If the ratio is greater than 50, it is likely that the urine is adulterated with the buprenorphine dipped directly in the urine [4, 5]. In these cases, norbuprenorphine is very low, approximately 10 ng/mL if the patient has not been taking buprenorphine for more than 4 days [29]. In addition, buprenorphine levels are often above the upper cut-off levels, which confirms an adulteration of the urine sample. However, high buprenorphine levels do not always indicate adulteration unless the norbuprenorphine level is extremely low because the high levels of buprenorphine can result from various reasons such as patients having a high metabolism or being dehydrated. Therefore, the high levels of buprenorphine should be used as confirmation, not for identification of adulteration. Instead, prescribers should focus on the ratio between buprenorphine and norbuprenorphine (>50) to accurately identify the adulteration of a UDS test [32]. If the ratio is >50, then prescribers calmly present the result to the patient and wait for them to talk about the result.
The next thing to focus on in the quantitative UDS results is the ratio between norbuprenorphine to creatinine. Creatinine here is used to “standardize” the metabolite levels because buprenorphine and norbuprenorphine levels can fluctuate, influenced by the hydration status and renal condition of the patient [3]. Also, when prescribers look at the ratio between norbuprenorphine and creatinine, they should pay attention to the units of these levels because buprenorphine and norbuprenorphine are often reported with (ng/mL), while creatinine is reported with (mg/dL). Prescribers can use the (*10-4) to adjust the ratio to make reading easier, which is used here henceforward.
If patients are on 8mg/day or more of buprenorphine, the ratio between norbuprenorphine to creatine is generally greater than 0.5 * 10-4 while if they are on 12mg/day or more, the ratio is usually above 1.5 * 10-4 [13, 14]. If the ratio between norbuprenorphine and creatinine is lower than these ratios, it is possible patients are not taking buprenorphine as prescribed. However, besides intentional non-adherence, there are many cases in which norbuprenorphine levels are low, and consequently, the ratio between norbuprenorphine and creatinine is low. Some of the cases include; 1) unintentional non-adherence such as forgetting to take the medication, 2) certain medical conditions such as liver disease that prevent the metabolism of buprenorphine, 3) medications that interfere with the buprenorphine metabolism in the system, and 4) slow metabolism in patients, which results in low metabolites in urine [14]. Prescribers can review the norbuprenorphine/creatinine ratio history to see if there are any noticeable changes, which might subtly indicate any behavioral or social changes in their patients.
Discussion
The following is the list of features that buprenorphine prescribers should pay attention to:
PDMP
- Controlled substances besides buprenorphine are prescribed.
- Maintenance buprenorphine dose is >24mg/day.
UDS
- Positive substances are detected besides buprenorphine and norbuprenorphine; buprenorphine and/or norbuprenorphine are not detected.
- Creatinine level is <20mg>
- The ratio between buprenorphine and norbuprenorphine is >50.
- For the patients whose daily buprenorphine dose is ≥8mg, the ratio of norbuprenorphine/creatinine is less than 0.5; for those with daily buprenorphine dose is ≥12mg, the ratio is <1>
The order of these concerning features as laid out above in the algorithm is not obligatory. Especially, the order in the UDS section of the algorithm is flexible since salient features might be easily noticeable in each laboratory result. It is beneficial, though, for novice prescribers to follow the order of the algorithm to ensure all concerning features are addressed. Although not all states mandate PDMP reviewing before prescribing controlled substances, by following the algorithm, patients’ safety and continuity of care are assured. Omitting the PDMP review or assuming, because the UDS report is negative for controlled substances, the PDMP would be as well, risks overlooking patients’-controlled substance use who are taking these substances intermittently.
If prescribers detect any of these features, they can educate their patients about them. Sublingual buprenorphine can be challenging to take because it must be dissolved completely under the tongue. Any acidic food or beverage immediately before the intake should be avoided, and there should be no smoking, eating, or drinking 30 minutes after the intake [11]. Therefore, education and encouragement of regular intake and how to take the medication should be re-discussed with patients.
In addition, there are general measures available to prescribers in case prescribers are suspicious that patients are diverting their medicine. SAMHSA recommends various strategies such as shorter visit intervals with more frequent urine tests and fewer prescriptions, observed medication intake, and as final recourses, altering the treatment modality to a higher level of care, such as Intensive Outpatient Program (IOP), detox/inpatient where they can be monitored 24/7, and prescription change to long-acting buprenorphine injection or methadone. Call-back options can be done but patients can store buprenorphine medication elsewhere while bringing the empty film covers, so it is doubtful how effective this strategy can be to monitor their adherence [25].
Finally, these features can be incorporated into the EMR as a clinical decision support system, which would result in potential enhancements for better monitoring with the interoperability of EMRs, PDMPs, and UDS laboratory systems. For example, urinary creatine levels <20mg>50 is easily noticeable due to unusually low norbuprenorphine levels; however, the norbuprenorphine/creatinine ratio could be hard to calculate. It would be an improvement for EMRs to have interoperability with the laboratory results and demonstrate the norbuprenorphine/creatinine ratio, ideally, displayed in a chronological line graph as a clinical decision support tool, so that prescribers can easily understand any fluctuation of the ratio levels and identify the behavioral (in-)consistency of buprenorphine intake.
Conclusion
The algorithm that this paper proposes is a general process of monitoring an OUD patient’s treatment progress. It is a simplified and generalized protocol for buprenorphine treatment monitoring. There could be many exceptions in this algorithm, so it must be tailored to each patient. The best use of this algorithm is to understand the patients better. This allows patient-centered treatment, searching together for a better treatment plan and an optimal outcome.
Figure 1: Monitoring Algorithms with Suggested Measures.
References
- National Institute on Drug Abuse, Advancing Addiction Science.
Publisher | Google Scholor - (2024). White House Releases List of Actions Taken by the Biden-Harris Administration Since January 2021 to Address Addiction and the Overdose Epidemic
Publisher | Google Scholor - (2024). Missouri Implements Statewide Prescription Drug Monitoring Program. Office of Administration Commissioners’ Office.
Publisher | Google Scholor - D'Souza RS, Lang M, Eldrige JS. (2024). Prescription Drug Monitoring Program. [Updated 2023 Jun 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
Publisher | Google Scholor - Islam MM, McRae IS. (2014). An inevitable wave of prescription drug monitoring programs in the context of prescription opioids: pros, cons and tensions. BMC Pharmacol Toxicol., 15:46.
Publisher | Google Scholor - (2014). Substance Abuse and Mental Health Services Administration. Waiver Elimination (MAT Act).
Publisher | Google Scholor - (2024). Prescription Drug Monitoring Programs.
Publisher | Google Scholor - FDA Drug Safety Communication: FDA urges caution about withholding opioid addiction medications from patients taking benzodiazepines or CNS depressants: careful medication management can reduce risks.
Publisher | Google Scholor - American Psychiatric Association, DSM-5 Task Force. (2013). Diagnostic and statistical manual of mental disorders: DSM-5™ (5th ed.). American Psychiatric Publishing, Inc.
Publisher | Google Scholor - Buprenorpine Quick Start Guide. (2024). SAMHSA: Substance Abuse and Mental Health Services Administration.
Publisher | Google Scholor - (2016). Substance Abuse and Mental Health Services Administration (SAMHSA). Medications for opioid use disorder.
Publisher | Google Scholor - Greenwald MK, Johanson CE, Moody DE, et al. (2003). Effects of buprenorphine maintenance dose on mu-opioid receptor availability, plasma concentrations, and antagonist blockade in heroin-dependent volunteers. Neuropsychopharmacology. 28(11):2000-2009.
Publisher | Google Scholor - Furo H, Schwartz DG, Sullivan RW, Elkin PL. (2021). Buprenorphine Dosage and Urine Quantitative Buprenorphine, Norbuprenorphine, and Creatinine Levels in an Office-Based Opioid Treatment Program. Subst Abuse. 15:11782218211061749.
Publisher | Google Scholor - Furo H, Wiegand T, Rani M, Schwartz DG, Sullivan RW, Elkin PL. (2023). Association Between Buprenorphine Dose and the Urine Norbuprenorphine to Creatinine Ratio. Subst Abuse, 17:11782218231153748.
Publisher | Google Scholor - Ferri M, Finlayson AJ, Wang L, Martin PR. (2014). Predictive factors for relapse in patients on buprenorphine maintenance. Am J Addict., 23(1):62-67.
Publisher | Google Scholor - White LD, Hodge A, Vlok R, Hurtado G, Eastern K, Melhuish TM. (2018). Efficacy and adverse effects of buprenorphine in acute pain management: systematic review and meta-analysis of randomised controlled trials. Br J Anaesth, 120(4):668-678.
Publisher | Google Scholor - Caritis SN, Bastian JR, Zhang H, et al. (2017). An evidence-based recommendation to increase the dosing frequency of buprenorphine during pregnancy. Am J Obstet Gynecol., 217:459.e1-459.e6.
Publisher | Google Scholor - Martin CE, Shadowen C, Thakkar B, Oakes T, Gal TS, Moeller FG. (2020). Buprenorphine dosing for the treatment of opioid use disorder through pregnancy and postpartum. Curr Treat Options Psychiatry, 7:375-399.
Publisher | Google Scholor - Motil KJ, Montandon CM, Garza C. (1990). Basal and postprandial metabolic rates in lactating and nonlactating women. Am J Clin Nutr., 52(4):610-615.
Publisher | Google Scholor - Butte NF, Hopkinson JM, Mehta N, Moon JK, Smith EO. Adjustments in energy expenditure and substrate utilization during late pregnancy and lactation. Am J Clin Nutr, 1999;69(2):299-307.
Publisher | Google Scholor - Gunderson EP. (2014). Impact of breastfeeding on maternal metabolism: implications for women with gestational diabetes. Curr Diab Rep., 14(2):460.
Publisher | Google Scholor - Buresh M, Ratner J, Zgierska A, Gordin V, Alvanzo A. (2020). Treating Perioperative and Acute Pain in Patients on Buprenorphine: Narrative Literature Review and Practice Recommendations. J Gen Intern Med, 35(12):3635-3643.
Publisher | Google Scholor - Oakley B, Wilson H, Hayes V, Lintzeris N. (2021). Managing opioid withdrawal precipitated by buprenorphine with buprenorphine. Drug Alcohol Rev., 40(4):567-571.
Publisher | Google Scholor - Seguí HA, Melin K, Quiñones DS, Duconge J. (2020). A review of the pharmacogenomics of buprenorphine for the treatment of opioid use disorder. J Transl Genet Genom., 4:263-277.
Publisher | Google Scholor - Jeon S, Kim KH, Yun CH, et al. (2008). An NH2-terminal truncated cytochrome P450 CYP3A4 showing catalytic activity is present in the cytoplasm of human liver cells. Exp Mol Med., 40(2):254-260.
Publisher | Google Scholor - McCance-Katz EF, Sullivan LE, Nallani S. (2010). Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict, 19(1):4-16.
Publisher | Google Scholor - McCance-Katz EF, Moody DE, Prathikanti S, Friedland G, Rainey PM. (2011). Rifampin, but not rifabutin, may produce opiate withdrawal in buprenorphine-maintained patients. Drug Alcohol Depend, 118(2-3):326-334.
Publisher | Google Scholor - Grande LA, Cundiff D, Greenwald MK, Murray M, Wright TE, Martin SA. (2023). Evidence on Buprenorphine Dose Limits: A Review. J Addict Med, 17(5):509-516.
Publisher | Google Scholor - Tzatzarakis MN, Vakonaki E, Kovatsi L, et al. (2015). Determination of buprenorphine, norbuprenorphine and naloxone in fingernail clippings and urine of patients under opioid substitution therapy. Journal of Analytical Toxicology, 39(4):313-320.
Publisher | Google Scholor - Kronstrand R, Nyström I, Andersson M, et al. (2008). Urinary detection times and metabolite/parent compound ratios after a single dose of buprenorphine. J Anal Toxicol., 32:586-593.
Publisher | Google Scholor - Swotinsky RB. (2021). The Medical Review Officer’s Manual: MROCC’s Guide to Drug Testing. Sixth edition. OEM Press.
Publisher | Google Scholor - Suzuki J, Zinser J, Issa M, Rodriguez C. (2017). Quantitative testing of buprenorphine and norbuprenorphine to identify urine sample spiking during office-based opioid treatment. Subst Abuse, 38:504-507.
Publisher | Google Scholor - Furo H, Whitted M, Lin T, et al. (2024). Buprenorphine, norbuprenorphine, and naloxone levels in adulterated urine samples: can they be detected when buprenorphine/naloxone film is dipped into urine or water? Substance Use: Research and Treatment, 18.
Publisher | Google Scholor - Weigand TJ. (2016). Use and interpretation of buprenorphine metabolite profiles during maintenance treatment. Poster Presented at: 47th American Society of Addiction Medicine (ASAM) Annual Conference, 14-17.
Publisher | Google Scholor - Kumar R, Viswanath O, Saadabadi A. Buprenorphine. (2023). In: StatPearls Treasure Island (FL): StatPearls Publishing.
Publisher | Google Scholor