Research Article
Mineralization of Dolomite
1Department of Geology, Sri J.N.M.PG College, Lucknow, India.
2Department of chemistry Sri JNMPG College Lucknow U.P.India.
*Corresponding Author: D.K. Awasthi, Department of Chemistry, Sri J.N.M.PG College, Lucknow UP, India.
Citation: Anshumali S., D.K. Awasthi. (2023). Mineralisation of Dolomite. Clinical and Laboratory Research, BRS Publishers. 1(1); DOI: 10.59657/clr.brs.23.005
Copyright: © 2023 D.K. Awasthi, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: July 01, 2023 | Accepted: July 17, 2023 | Published: July 24, 2023
Abstract
Worldwide carbonate rocks are occurring abundantly. These carbonate rocks are a major class of sedimentary rocks group. Carbonate are sedimentary rocks formed at (or near) the Earth surface by precipitation from solution at surface temperatures. The two broad categories are limestone, which is composed of calcite or aragonite (different crystal forms of CaCO3) and dolostone, which is composed of the mineral dolomite (Ca Mg (CO3)2). Dolomite is not a simple mineral; it can have a variety of origin, can form as a primary precipitate, a diagenetic replacement, or as a hydrothermal /metamorphic phase, all that it requires is permeability, a mechanism that facilitates fluid flow, and a sufficient supply of magnesium. Dolomite can also form in lakes, on or beneath the shallow seafloor, in zones of brine reflux, and in early to late burial settings. It may form from seawater, from continental waters, from the mixing of basial brines, the mixing of hypersaline brine with seawater, or the mixing of seawater with meteoric water, or via the cooling of basial brines. Potential fluid sources are seawater and subsurface fluid of marine and/or meteoric origin: and addition Mg could be released from high-Mg calcite and smectite clays. The only abundant source of Mg2+ ions for early diagenetic surface and near-surface dolomitization is seawater. Dolomitization also creates new crystals, with new rhomb growth following the dissolution of less stable precursors. Dolomitization model and formation depend on the source dolomitization site and lastly, there must exist a favorable condition for a chemical reaction. One particular type of dolomite which may be a cement or a replacement is baroque dolomite, also called 'saddle' or 'white sparry' dolomite and known to mineral collectors as pearl spar. It is characterized by a warped crystal lattice.
Keywords: carbonates; dolomite; calcite; fluid source; dolomitization
Introduction
Carbonate rocks are a class of sedimentary rocks composed primarily of carbonate minerals. Carbonate are sedimentary rocks formed at (or near) the Earth surface by precipitation from solution at surface temperatures. The two major types are limestone, which is composed of calcite or aragonite (different crystal forms of CaCO3) and dolostone, which is composed of the mineral dolomite (CaMg(CO3)2). Carbonate rocks are depositional most simple but diagenetically most complex rocks of the world. There are a number of carbonate minerals which are formed by combining of one or more ion with the CO3 2- ion. Dolomite is an anhydrous carbonate mineral composed of calcium magnesium carbonate, ideally CaMg(CO3)2. The term is also used for a sedimentary carbonate rock composed mostly of the mineral dolomite. An alternative name sometimes used for the dolomitic rock type is dolostone. Dolomite is an unusual carbonate mineral. It is common in ancient platform carbonates, yet it is rare in Holocene sediments Table 1 [1].
Table 1
| Mineral name | Chemical formula |
| Calcite | Caco3 |
| Aragonite | Caco3 |
| Dolomite | CaMg(co3)2 |
| Ankerite | CaFe(co3)2 |
| Magnesite | Mgco3 |
| Siderite | Feco3 |
Table 1 Some important minerals the mineral dolomite is the main component of the sedimentary rock that is also called Dolomite. It is similar to the mineral calcite but contains magnesium as well as calcium. Just based on looks, the rock dolomite is often impossible to distinguish from limestone, which is composed mainly of calcite. Dolomite crystals are usually rhombic-shaped but, unlike most other minerals, the crystal faces typically are curved.
Chemical compound: calcium magnesium carbonate.
Chemical formula: CaMg(CO3)2 (Ca = calcium, Mg = magnesium, C = carbon, O = oxygen).
Color: white, gray, greenish, brown, pink.
Luster: often glassy.
Hardness: 3.5–4.
Specific gravity: 2.8.
Amount of transparency: transparent to translucent
Dolomite is a very common mineral, and is known for its saddle-shaped curved crystal aggregates. A unique, isolated Dolomite occurrence in Enugu, Spain has provided colorless transparent crystals that resemble the Iceland spar variety of calcite The occurrence of Kolwezi, in the Congo, has produced some fascinating, cobalt-rich specimens that are a beautiful hot pink colour and highly popular.
Dolomite forms in a different crystal grass differing from the calcite group minerals. This can be noted by the fact that Dolomite generally forms more elongated crystals than those of the Calcite group. In addition, Dolomite never occurs in scalenohedral crystals, whereas minerals of the Calcite group do.
Dolomite
Table 1
| Composition | Calcium magnesium carbonate. The amount of calcium and magnesium in most specimens is equal, but occasionally one element may have a slightly greater presence than the other. Small amounts of iron and manganese are sometimes also present. |
| Variable Formula | (Ca,Mg)2(CO3)2;(Ca,Mg,Fe,Mn)2(CO3)2 |
| Color | Colorless, white, gray, peach, pink, yellow, and orange. Rarely yellow, green, red, and black. |
| Streak | White |
| Hardness | 3.5 - 4 |
3D Crystal Atlas (Click for animated model)
Dolomite types
Iron bearing dolomite
Dolomite
On the basis of the mode of formation, dolomites can broadly be divided into two groups: primary dolomite and secondary dolomite [2]. Primary dolomite precipitate directly from aqueous solution, mostly at or near room temperature (20-35oC), with no CaCO3 dissolution involved.3 However, dolomite can also form as a secondary phase replacing the precursor mineral calcite (dolomitization process). The widely accepted hypothesis of dolomitization is that limestone is transformed into dolomite by the dissolution of calcite followed by dolomite precipitation [4]. That dolomite grows at the expense of calcite was demonstrated by the formation of dolomite on the edges and corners of calcite during dolomitization [5]. During dolomitization dissolution of precursor mineral calcite will supply the much-needed Ca+2 wherein Mg+2 (and CO3-2) is supplied by the dolomitizing fluid [6].
Formation of dolomite
Dolomite is formed by the replacement of the calcite ions by the magnesium ions. Depending upon the ratio of the Mg ions in the crystal lattice they have different names Fig -1. Modern dolomite formation has been found to occur under anaerobic conditions in supersaturated saline lagoons in Brazil. In the high-Mg calcite, there is a 0-32 mol% of Mg substitution for Ca. In the protodolomite there is roughly 55-60 Mol
Source of magnesium ion
Prospective fluid sources are seawater and underground water of oceanic and/or meteoric source: while addition of magnesium can be added from high magnesium calcite and clays. Sea water is thought to be the prime source of magnesium ion for early diagenetic surface and near-surface dolomitization. It consists 1290 part per million magnesium (0.052 moles I-I) and 411 ppm Ca (0.01 moles I-I), i.e., an Mg/Ca weight ratio of. 3.14 or molar proportion of 5.2. Beside meteoric water generally inconstant possess low values of both; for example, normal river water has 15 ppm Ca and 4 ppm Mg depicting a molar Mg/Ca ratio of 0.44. Therefore, ocean water is usually thought to be the origin of the Mg2+ ions for maximum primary dolomitization but this water is generally altered in numerous dolomites forming models in trend [8].
Dolomitization model
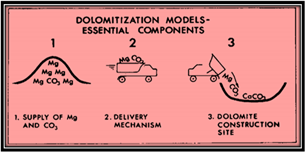
Dolomite is one of the complex minerals and its artificial occurrence is difficult to study in lab experimentations. Though it is a very well-ordered mineral and demanding phase to precipitate, yet still dolomite synthesis at sedimentary temperatures using natural waters has not shown. Controlling chemical factors on dolomite formation from water is problematic to clarify and largely have been concluded from high-temperature experimentations [9]. Figure 5 Essential elements of any dolomitization model, supply of Mg and the supply of carbonate must be sufficient to form the observed amount of dolomite there must be a delivery mechanism commonly in the form of fluid flow adequate to deliver these ions to the dolomitization site and lastly there must exist favourable condition for chemical reaction. For elucidation of primeval dolomite five extensive divisions of dolomitization model presently exists,
- Evaporative
- Seepage-reflux
- Mixing-zone
- Burial
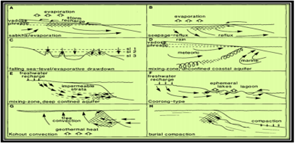
- Seawater models Figure 6 and 7.
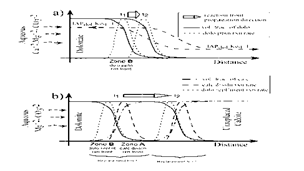
Figure 7 Conceptual models for (a) dolomite precipitation and (b) dolomite replacement scenarios. (a) The thick black lines are the volume fraction of dolomite that is precipitation from the invading solution from the left. The dashed lines represent the dolomite precipitation rates t1 and t2 represent two different time slices. Dolomite precipitation front is moving towards the right as indicated by the open arrow. (b) The replacement front consists of two reaction zones namely zone A and zone B. In Zone A calcite chemically dissolves first releasing Ca+2 in the aqueous solution and producing secondary porosity. The Ca+2 thus released will eventually supersaturate the aqueous solution and precipitate dolomite that will pressure dissolve an equal amount of calcite in zone B. Again, t1 and t2 represents two different time slices. Dolomite precipitation front is moving towards the right as indicated by the open arrow [10].
Figure 5: Essential elements of any dolomitization model.
Figure 6: Models of dolomitization, illustrating the variety of mechanisms for moving dolomitizing fluids through the sediments [1].
Figure 7: Conceptual models for (a) dolomite precipitation and (b) dolomite replacement scenarios.
Replacement dolomite
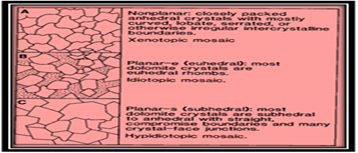
Formation of dolomite by replacement of CaCo3 ranges from fabric damaging to retaining and from fabric selective to persistent. The important aspects now are grain mineralogy and crystal extent, the timing of dolomitization and natural surroundings of dolomitizing liquids. Forms of dolomite crystals in replacement assortments differs from anhedral to euhedral shape, with the terminologies xenotropic and idiotypic denoting to the mosaics. Sibley [11] put stress on the origin of crystal boundary shapes, identifying non-planar, euhedral and subhedral categories Fig-8 and 9.
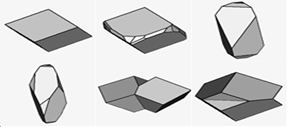
Figure 8: Three common dolomite textures; (A) Non-planar crystals in a xenotropic mosaic; (B) Planar-e crystals (e for euhedral) in an idiotypic mosaic; (C) Planar-s crystals (s for subhedral) in a hepatotropic mosaic.
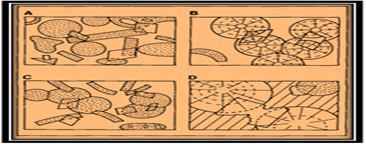
Figure 9: Common dolomite rhomb relationships in limestones; (A) Pre-compaction rhomb’s, grains in point contact. (B) Compaction after rhomb precipitation so that ghost textures in rhomb’s are displaced relative to grains; (C)Post-compaction rhomb’s including fractured grains and stylolite’s; (D) Pre-sprite rhomb’s etched and replaced [1].
Dolomite cement
Though dolomite is mainly a replacement, cement of this mineral is common. In Pleo-Pleistocene dolomites of the Caribbean-Bahamas, dolomite cement generally formed amongst dolomitized grains and in the spaces. The dolomite can also be found as clear spur, straight analogous to calcite spar, although usually cement is just a single layer of large rhomb’s in between the spaces of grains. Crystals are too clear and thus they are known as limpid for their white look. Stress has been put on these, proposing they are a distinctive creation of mixing-zone dolomitization. In few cases, dolomite cement comprises regions of syntaxial calcite depicting fluctuation in pore water chemistry (Figure 10) [12].
Figure 10: Terms for describing dolomite textures.
Arouse dolomite
This specific dolomite is also known as saddle or white sparry dolomite. Baroque dolomite is known to be forming as a result of replacement. In the minerals collecting community, it is known as pearl spar distinguished by a distorted crystal lattice it has bowed crystal looks, curved cleavage planes, having undulate extinction. Crystal of this dolomite which is larger than a millimetre is generally a compound of sub-crystals, resulting in stepped surface of the crystal. If the dolomite is of replacement origin fluid inclusions, and calcite relics will be present which provides the crystals a gloomy look in thin section and displays shining lustre in hand specimens. Baroque dolomite is calcium rich and usually having Fe content (up to 15 moles
Applications in Detail
Construction: Dolomite, in its crushed form, is used for producing concrete and cement. Its high hardness makes it a robust material for construction purposes.
Agriculture: Farmers use dolomite for agricultural pH control. The chemical industry uses the mineral dolomite in making magnesium salts.
Steel Industry: It serves as a flux for the smelting of iron and steel, helping to remove impurities and improve the quality and strength of the end product.
Ceramics: In ceramics, dolomite is used as a source of magnesia (MgO) and also in the creation of ceramics and glass.
Petroleum Industry: Dolomite rocks contain oil and natural gas reserves and serve as reservoir rocks in petroleum extraction.
Environmental Impact and Sustainability
While dolomite provides numerous benefits, its extraction and use come with environmental considerations. Open-pit mining can lead to habitat loss and air pollution. However, sustainable practices, such as phased recovery and reclamation of mined lands, are being implemented in many parts of the world to mitigate these impacts. Furthermore, dolomite can naturally capture carbon dioxide (CO2), which contributes to climate change mitigation efforts.
Dolomite in Science and Research
Recent scientific research focuses on dolomite’s potential in carbon sequestration. This involves using dolomite to capture and store carbon dioxide, offering a way to reduce greenhouse gas emissions. Dolomite’s role in the carbon cycle and its potential contribution to reducing atmospheric CO2 levels are currently under intense study, promising new avenues for combating climate change.
Conclusion
In conclusion, dolomite is a versatile and multifunctional material that plays a significant role in various industries worldwide. Its unique chemical and physical properties, combined with its global distribution, make it an indispensable resource. While its extraction and use have environmental impacts, the implementation of sustainable practices and its potential in climate change mitigation mark dolomite as a material of considerable interest in the future. It’s not just a rock – it’s a cornerstone of modern industry and could potentially be a significant part of the solution to our climate crisis. Dolomite is by formation a very complex which not only forms in the ocean by the simple addition of Mg but also forms as result of Vaporization and burial of magnesium-containing water upon reaction with limestone. Different dolomite types have their own history embedded within it. Baroque a specific category of dolomite which may be cement or a replacement is, also called 'white sparry' dolomite and identified to mineral accumulators as pearl spar. The criteria for choosing between models are not definitive as could be desired and many of these criteria have been interpreted in more than one way. A considerable measure of work and research is anticipating to be completed identified with dolomite, its development and dolomitization process.
References
- Tucker ME, Wright PV. (1990). Carbonate sedimentology. Oxford, United Kingdom: Blackwell. 482.
Publisher | Google Scholor - Pichler T, Humphrey JD. (2001). The formation dolomite in island-arc sediments due to gas-seawater-sediment interaction. Jour Sediment Res.;71(3):394-399.
Publisher | Google Scholor - Wells A. (1962). Recent Dolomite in the Persian Gulf. Nature, 194:274-275.
Publisher | Google Scholor - Weyl PK. (1965). Pressure solution and the force of crystallization—A phenomenological theory. Jour Geophys Res, 64:2001-2025.
Publisher | Google Scholor - Kaczmarek SE, Sibley DF. A. (2007) comparison of nanometre-scale growth and dissolution features on natural and synthetic dolomite crystals: implications for the origin of dolomite. Jour Sediment Res., 77(5):424-432.
Publisher | Google Scholor - Merino E, Canals A. (2011). Self-accelerating dolomite for calcite replacement: self-organized dynamics of burial dolomitization and associated mineralization. Amer Jour Sci., 311(7):573-607.
Publisher | Google Scholor - Sibley DF. (1982). The origin of common dolomite fabrics. J sedim Petrol., 52:1987-1100.
Publisher | Google Scholor - Patterson RI,Kinsman DJJ. (1982). Formation of diagenetic dolomite in coastal sabkhas along the Arabian (Persian) Gulf. Bull Am Ass petrol Geol, 66:28-43.
Publisher | Google Scholor - Zenger DH. (1972). Dolomitization and unifonnitarianism. J geol Educ, 20(3):107-124.
Publisher | Google Scholor - Banerjee A. (2016). Estimation of dolomite formation: Dolomite precipitation and dolomitization. Journal of the Geological Society of India., 87(5):561-572.
Publisher | Google Scholor - Sibley DF, Gregg JM. (1987). Classification of dolomite rock texture. J sedim Petrol., 57:967-975.
Publisher | Google Scholor - Folk RL, Land LS. (1975). Mg/Ca ratio and salinity, two controls over crystallization of dolomite. Bull Am. 1975;59(1):60-68.
Publisher | Google Scholor - Radke BH, Mathis RL. (1980). On the formation and occurrence of saddle dolomite. I sedim Petrol, 50:1149-1168.
Publisher | Google Scholor