Case Report
Management Massive Acute Thrombosis of a Mechanical Mitral Valve in A Young Patient on Acenocoumarol: Surgery Versus Systemic Thrombolysis
- Said Khallikane 1*
- Bassam Bencharfa 2
- Noureddine Atmani 3
- Abdessamad Abdou 4
- Ayoub Belhadj 5
- Younes Aissaoui 6
1Former Anesthesiologist, Professor of Anesthesia-Intensive Medicine, Cardiothoracic Anesthesiologist Cardiothoracic Anesthesia-Cardiovascular ICU, Anesthesiology-ICU-Emergency Department, Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Kingdom of Morocco.
2Anesthesiologist-Intensivist, Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Kingdom of Morocco.
3Cardiothoracic and Vascular Surgeon, Professor of Cardiac Surgery, mini-invasive Cardiac Surgery, Interventional Cardiovascular Surgery, Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 50000-Sidi Mohamed Beni Abdellah University, Faculty of Medicine and Pharmacy of Fes, Kingdom of Morocco.
4Head of Cardiothoracic and Vascular Surgeon, Professor of Cardiac Surgery, mini-invasive Cardiac Surgery, Interventional Cardiovascular Surgery, Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Kingdom of Morocco.
5Head of Surgical ICU, Professor of Anesthesiology-Critical Care, Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Kingdom of Morocco.
6Head of Medical ICU, Professor of Anesthesiology-Critical Care, Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Kingdom of Morocco.
*Corresponding Author: Said Khallikane, Anesthesiology-ICU-Emergency Department, Avicenna Military Hospital, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech 40000, Kingdom of Morocco.
Citation: Khallikane S., Bencharfa B., Atmani N., Abdou A., Belhadj A., et al. (2025). Management Massive Acute Thrombosis of a Mechanical Mitral Valve in A Young Patient on Acenocoumarol: Surgery Versus Systemic Thrombolysis, Journal of Clinical Cardiology and Cardiology Research, BioRes Scientia Publishers. 4(1):1-16. DOI: 10.59657/2837-4673.brs.25.047
Copyright: © 2025 Said Khallikane, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: January 27, 2025 | Accepted: March 11, 2025 | Published: March 21, 2025
Abstract
Mechanical mitral valve thrombosis is a severe complication that can occur in patients with mechanical heart valves, particularly when anticoagulation therapy is not adequately managed. In patients on acenocoumarol, a commonly used vitamin K antagonist, the balance between preventing thromboembolic events and avoiding hemorrhagic complications is critical. When mechanical mitral valve thrombosis presents with hemodynamic instability and respiratory failure, urgent management is required.
In our patient, emergency surgery was chosen after thrombolysis failure and confirmed valve thrombosis, through sternotomy and cardiopulmonary bypass (CPB), the mitral valve was cleared of thrombi and restored to its anatomical position after ensuring the complete release of the leaflets and the absence of any intraventricular thrombus, with successful weaning from cardiopulmonary bypass and a brief improvement in hemodynamic parameters. However, the patient subsequently developed septic shock originating from the lungs and unfortunately passed away. Valve thrombosis has multifactorial causes and often presents with nonspecific symptoms. Effective prevention relies on meticulous anticoagulation, while surgery remains a last-resort option. Comprehensive management requires a multidisciplinary approach, integrating anticoagulation, thrombolytic therapy, and surgical intervention when necessary. Patient education and individualized treatment plans are essential. Future research should prioritize the development and evaluation of novel anticoagulants to improve outcomes. This clinical scenario poses a challenge in deciding between thrombolysis and surgical intervention. The aim of this review is to explore the literature on the management strategies for mechanical mitral valve thrombosis in such patients, comparing the benefits and risks of surgery versus thrombolysis.
Keywords: mechanical mitral valve thrombosis; vitamin k antagonist; hemodynamic instability; thrombolysis failure; thrombectomy
Introduction
Thrombosis of mechanical heart valves is rare but serious, with an incidence of 0.3% to 6.2%, and poses a significant concern due to its life-threatening nature. Managing mechanical mitral valve thrombosis (MMVT), particularly with hemodynamic instability, is challenging, requiring a balance between the risks of thrombolysis and potential complications from surgery, such as high mortality and embolic events. Despite the increased thrombosis risk, mechanical valve replacement is often preferred in resource-limited settings. Mechanical valves, which make up 66% of prosthetic valves in the U.S., are durable but susceptible to thrombosis. With over 1 million valve replacements performed globally each year, improving strategies to prevent and manage these complications is essential [1]. The development of mechanical prosthetic mitral valves has improved life expectancy in patients with rheumatic heart disease, but thrombosis remains a serious complication, potentially leading to conditions like pulmonary edema, ventricular failure, and cardiogenic shock. Treatment options—medical therapy, thrombolytic therapy, or surgical thrombectomy/replacement—depend on the patient's hemodynamic stability and risk-benefit evaluation. Managing thrombosis in shock is particularly challenging due to factors like thrombosis duration, bleeding risks, and incomplete clinical information. Systemic thrombolysis can be life-saving but carries high risks of hemorrhage, creating a dilemma between surgery and thrombolysis. Post-surgery management includes controlled heparinization, oral anticoagulation, and regular INR monitoring. This case illustrates the use of thrombolysis followed by emergency surgery for mechanical mitral valve stenosis in a young patient, highlighting challenges in anticoagulation, hemodynamics, and ventilation in high-risk settings [2,3].
Mechanical mitral valve thrombosis (MMVT) is a critical condition with high mortality and morbidity [4]. This case involved a young woman with severe prosthetic valve dysfunction, hemodynamic instability, and multiple thrombi due to a subtherapeutic INR despite long-term acenocoumarol therapy. Despite initial thrombolysis, persistent issues necessitated high-risk surgery. The case underscores the importance of individualized management strategies, particularly in resource-limited settings, and highlights the need for adaptable, patient-centered approaches to effectively manage MMVT. that has accrued from the heart team's multidisciplinary management and to look for the optimal treatment approach. Despite increasing success with thrombolytic therapy, surgical removal of left mitral valve thrombi remains widely performed globally. This article highlights key surgical and thrombolytic treatment options for managing left mitral valve thrombi. Comprehensive management, involving both medical and surgical teams, is crucial for high-risk patients emphasizing a multidisciplinary approach to optimize outcomes [2,3].
Case Report
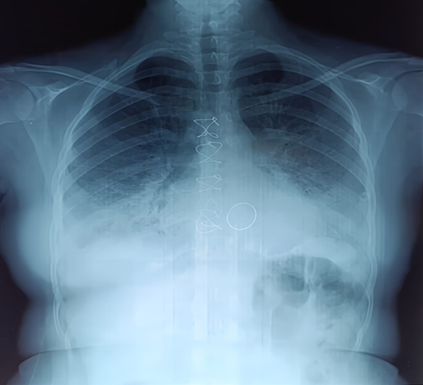
A 22-year-old female with rheumatic valvular heart disease, post-mitral valve replacement and tricuspid valve repair, presented with progressive dyspnea and hemoptysis. One year after surgery, she was found to have a thrombus on her mechanical mitral valve. She was treated with aspirin and heparin, resulting in improved transvalvular gradient and partial thrombus resolution. Following stabilization, she was discharged on acenocoumarin with regular anticoagulation monitoring. Two years post-surgery, the patient presented with severe global congestive heart failure, hypoxemia, and dyspnea. Clinical examination showed pulmonary crackles, mild hypoxia, hypotension, tachycardia, and tachypnea. A chest X-ray revealed cardiomegaly and bilateral pulmonary edema (Figure 1).
Figure 1: Preoperative frontal chest X-ray showing bilateral basal opacities with blunting of both pleural recesses, a normal-appearing cardiac silhouette, sternal wire sutures, and the projection of the mechanical valve ring.
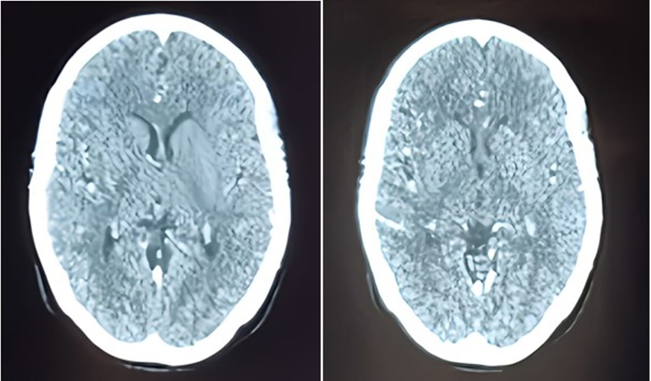
Blood tests showed leukocytosis, elevated NT-proBNP levels (800), and moderate metabolic acidosis. Transthoracic echocardiography revealed near-complete obstruction of the mechanical mitral valve with a high transvalvular gradient, significant regurgitation, and no thrombus in the left atrium. The patient was initially stabilized with norepinephrine, heparin, diuretics, and dual antiplatelet therapy. However, on the fifth day, her condition worsened with shock, hypoxia, and confusion, raising concerns about systemic embolism, including potential cerebral involvement. A brain CT scan ruled out cerebral embolism (Figure 2).
Figure 2: Contrast brain CT scan in axial slices, performed to investigate delayed thromboembolic complications, returned without abnormalities.
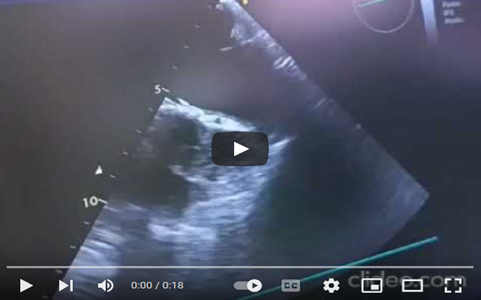
The patient's condition worsened despite initial stabilization with norepinephrine, dobutamine, and diuretics. Transthoracic echocardiography revealed severe pulmonary hypertension, a distended left atrium, high transvalvular gradient, significant mitral regurgitation, and a hyperkinetic left ventricle. She was administered 35 mg of Tenecteplase (TNK-tPA) and placed on high-flow oxygen therapy. After 8 hours of no improvement, she was taken to the operating room for thrombectomy, with INR of 2.03, and received vitamin K, exactly, vancomycin, and heating blanket. Anesthesia was induced with fentanyl, etomidate, and rocuronium, maintained with sevoflurane, and analgesia provided with fentanyl boluses. Valve debridement and declotting were performed, guided by transesophageal echocardiography (TEE), instead of valve replacement. TEE showed no atrial thrombus, enabling direct surgery. Pre-sternotomy TEE guided surgical planning, and Doppler ultrasound at the Scarpa's triangle confirmed patency of the femoral vein and artery for peripheral cannulation, while the biatrial view in TTE was prepared for the advancement of the venous cannula (Video 1).
Video 1: ETT in bicaval view to visualize the right atrium in contact with the probe, the superior vena cava containing the central venous catheter, and the inferior vena cava.
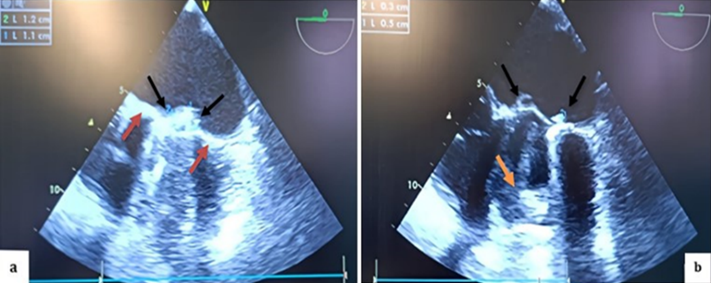
During surgery, it was noted that the common femoral artery was too small for the available venous cannulas, so central cannulation was chosen instead. The left ventricle (LV) was small, with one mitral valve leaflet restricted and the other completely obstructed, both exhibiting thrombi. The LV’s anterior and inferior walls retained contractility. The right ventricle was dilated, and the aortic ring appeared normal. A leaking annuloplasty was observed in the right atrium, while the left atrium, tricuspid valve, and right ventricle were free of thrombi. In the mid-esophageal view, the St. Jude mechanical valve showed a thickened ring with thrombi on both sides (Figure 3).
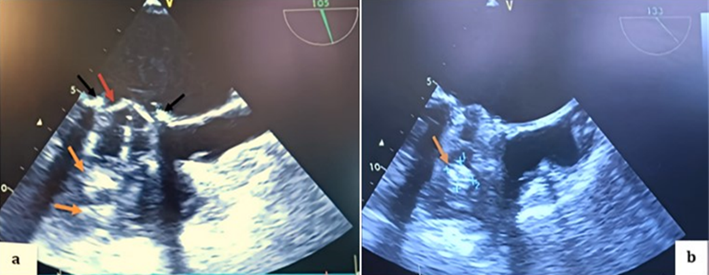
Figure 3: TEE in mid-esophageal view, transducer at 0 degrees, showing a thickened appearance of the St. Jude mechanical valve ring in an anatomical anti-position (a) (red arrow) with multiple thrombi on the atrial (a; b) (black arrow) and ventricular (b) (orange arrow) sides of both the internal and external leaflets.
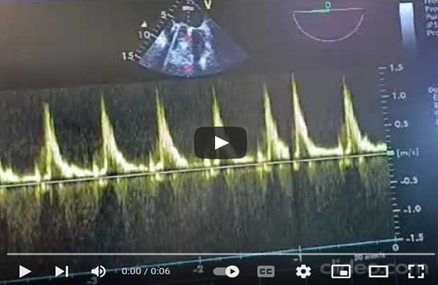
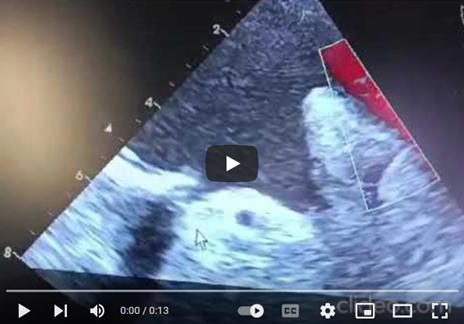
Pulsed Doppler TEE at the mechanical mitral valve in the mid-esophageal view, at a perpendicular plane of 60 degrees, showing a holosystolic retrograde mitral flow velocity of 1.3 m/s. (video 2).
Video 2: Pulsed Doppler TEE at the mechanical mitral valve in the mid-esophageal view, with the transducer at 60 degrees perpendicular to the flow, showing a holosystolic retrograde mitral flow velocity of 1.3 m/s.
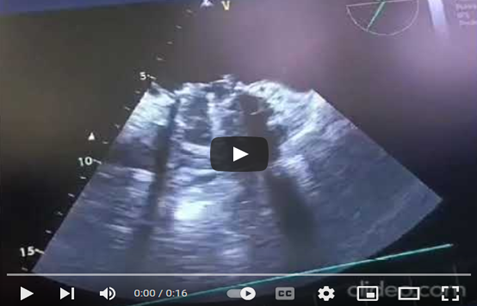
At 45° counterclockwise rotation, a dilated left atrium and an obstructed mechanical valve are observed, with multiple thrombi on the leaflets, ring, and core. There is no leaflet opening beneath the appendage, and limited movement is seen on the opposite side, reverberation echoes obscure most of the left ventricle walls, except for the anterior and inferior walls, which maintain preserved kinetic activity. (Video 3) 2D TEE in a mid-esophageal view at 105-to-135-degree angle, showing thrombi on atrial and ventricular side of mechanical valve, (Video 4).
Video 3: In the mid-esophageal view using 2D TEE, the left atrium was dilated, and a mechanical valve showed multiple mobile thrombi obstructing the leaflets, ring, and core. The leaflet opening was absent beneath the appendage, and the posteromedial leaflet had limited movement. The valve ring appeared thickened, filled with a hyper-echoic fibrinous thrombus, and reverberation echoes obscured the lateral and septal walls of the left ventricle. However, the anterior and inferior walls of the left ventricle maintained their kinetic activity.
Video 4: In the mid-esophageal four-chamber view at 35 cm from the dental arches, with slight counterclockwise rotation and centered on the left cavities, the TEE revealed a dilated left atrium in contact with the probe, thickened mitral valve leaflets with very limited movement. There was blockage of the leaflet at the junction of the lateral wall of the left atrium and left ventricle, accompanied by multiple hyper-echoic thrombi in the left ventricle, slightly obscured by reverberation echoes.
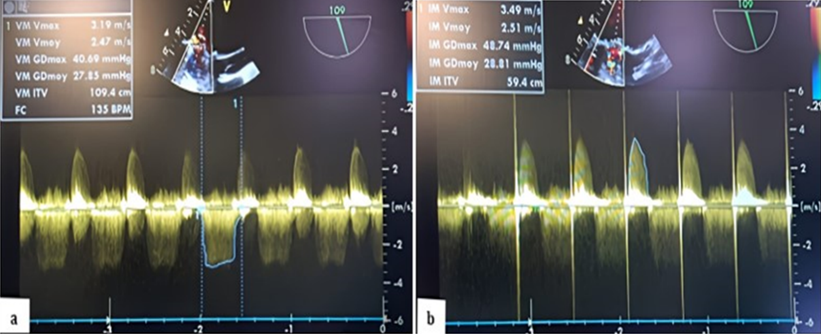
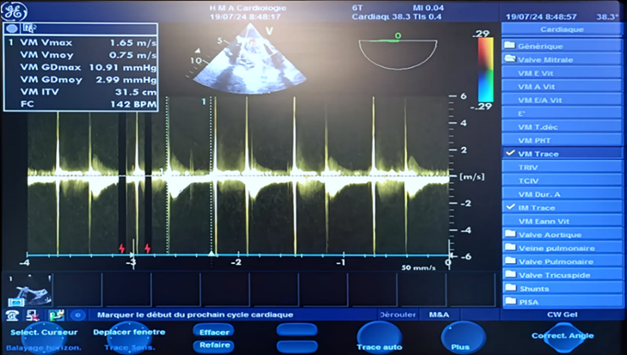
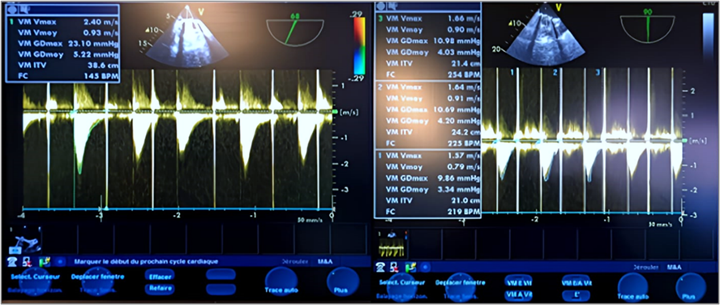
In color Doppler mode, TEE shows significant mitral regurgitation extending into the left atrium, the mean transvalvular gradient was 27.85 mm Hg with a VTI of 109.4 cm, indicating stenosis associated with severe mitral regurgitation was present, with a gradient of 28.80 mm Hg. (Figure 4,5) (Video 5).
Figure 4: TEE 2D in the mid-esophageal view, transducer at 105 degrees (a; b), showing thrombi on the atrial side of both the internal and external leaflets of the mechanical valve on the aortic valve side as well as on the opposite side (black arrows), with a thickened appearance of the mechanical valve ring (red arrow) and multiple thrombi on the ventricular side (orange arrows).
Figure 5: TEE Doppler with the transducer at 105 degrees showing a very high mean transvalvular gradient of 27.85 mm Hg on continuous Doppler, with a calculated valve area of 109.4 cm² (a) in favor of stenosis, and severe thrombotic regurgitation with a mean gradient of 28.80 mm Hg (b) in favor of severe mitral regurgitation.
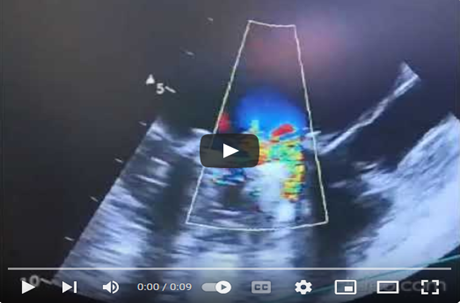
Video 5: TEE in the mid-esophageal view, with the transducer at 135 degrees, in color Doppler mode centered on the thrombosed mechanical mitral valve, showing significant mitral regurgitation reaching the depth of the left atrium.
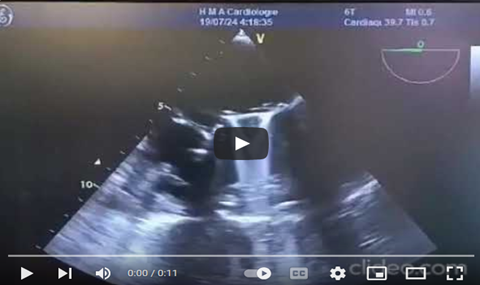
At 110-130°, a biatrial view showed the interatrial septum, left atrium, aorta, pulmonary pathway, aortic root, right atrium, and pulmonary artery. At 30 cm, the aortic root appeared differently, and sinuses were analyzed at 45°. The 135° cut displayed the ascending aorta. A counterclockwise rotation revealed a horn-shaped left auricle free of thrombus. Doppler imaging showed biphasic flow and negative flow during ventricular systole. The left superior pulmonary vein (LSPV) was seen above the left atrium with continuous red flow, and pulsed Doppler revealed positive S and D waves with no A wave during atrial systole (Video 6,7).
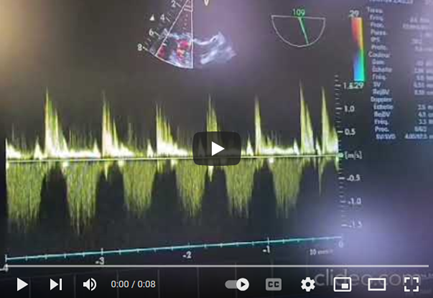
Video 6: Continuous Doppler TEE at the mechanical mitral valve showing high-velocity negative flow with a significant mean transvalvular gradient during diastole, along with a significant positive holosystolic regurgitant flow during systole of high velocity, indicating stenosis and significant valve leakage due to obstructive thrombosis.
Video 7: ETT in high esophageal view, a counterclockwise rotation and a cut plane between 45 and 60 degrees revealed a horn-shaped left auricle with a pointed bottom, free of thrombus.
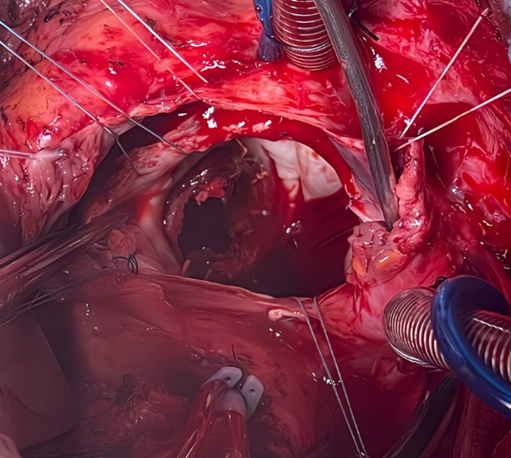
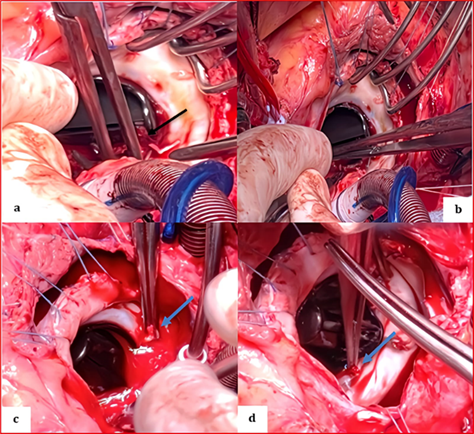
The chest was surgically reopened through division of the prosthetic sternum using an electric saw, and the thoracic cavity was carefully dissected. Cardiopulmonary bypass was initiated by the full heparinization of the patient. The circulating blood volume was mobilized, and the bypass was cannulated by venous drainage of the right atrium and aortic return. After clamping and opening the ascending aorta, both atriotomies were extended to the mitral orifice to allow a clear overview and manual control. Vancomycin was administered, and anesthesia was maintained with propofol and fentanyl. Cardiopulmonary bypass (CPB) was established with cannulas inserted into the aorta and veins. Heparin was readministered, according to activated clotting time (ACT) values measured every 15 minutes. Normothermic CPB was initiated, with ventilation reduced during bypass and halted during full-flow CPB. During surgery, the surgeon immobilized the mitral prosthesis and performed a small incision on the obstructing thrombus attached to one of the disc loops (Figure 6). The thrombus was flushed using sterile saline (Figure 7,8).
Figure 6: Surgical image after right arteriotomy and expanded transseptal approach at the roof of the left atrium showing the appearance of the St. Jude mechanical valve completely thrombosed, encompassing the ring, both leaflets, and the core.
Figure 7: Perioperative image showing the St. Jude mechanical valve partially cleaned in its initial anatomical anti-position during the extraction of remaining thrombi using forceps on the atrial side (a) (black arrow), the ventricular side (b) on the internal and external leaflets, and at the level of the coated ring (c, d) (blue arrows).
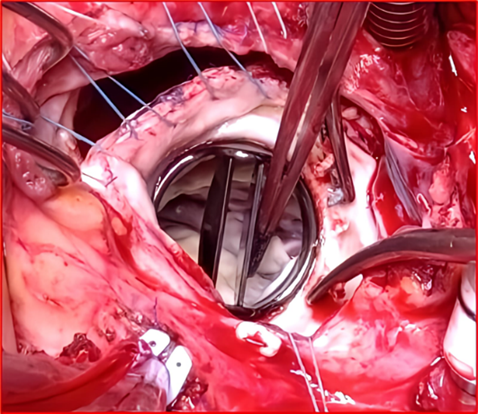
Figure 8: Surgical image showing a clean appearance of the St. Jude Medical mitral valve after being completely cleared of thrombi following saline washing, returned to its anatomical position, and opened freely around its pivot with both the anterior and posterior leaflets.
Intraoperative assessment confirmed no need for valve replacement, and oxygen delivery was adequate. The hematocrit was 20, and 2 units of red blood cells were transfused. Mean arterial pressure was maintained between 65-75 mm Hg. The left atrium was closed with debubbling, and after unclamping the aorta, continuous venting and defibrillation electrodes were placed. The clamping duration was 90 minutes, and post-clamp, hemodynamic stability returned within two minutes. CPB weaning was gradual, with dobutamine and norepinephrine support. After decannulation, blood was retrieved from the reservoir due to hypovolemia. Heparin was reversed, and hemostasis was verified before thoracic closure. The patient was successfully weaned from bypass, and post-CPB TEE showed good valve function, strong ventricular contractility, and improved filling pressures. CPB lasted 125 minutes (Video 8) (Figure 9).
Video 8: In the high esophageal view with color Doppler, a counterclockwise rotation between 35 and 60 degrees revealed red flow during systole and blue flow during diastole, indicating elevated filling pressure. Continuous-wave Doppler showed biphasic flow with no ECG P wave but elevated filling pressure, and negative flow during ventricular systole. The left superior pulmonary vein (LSPV) was visible above the left atrium with continuous red flow approaching the probe. Pulsed Doppler showed biphasic flow, including a positive S wave at 55 cm/s, a positive D wave at 37 cm/s, and no A wave during atrial systole. A spur was seen between the LSPV and auricle, not to be confused with a neoformation.
Figure 9: Postoperative continuous Doppler TEE on the mechanical mitral valve after weaning from CPB, showing a low mean gradient measured at 3 mm Hg following cleaning of the valve.
The patient returned to the intensive care unit with moderate doses of norepinephrine and dobutamine with the output of the surrounding drainage systems and a significant improvement in the hemodynamic parameters. Vital parameters, oxygenation, and cardiac function were continuously monitored. A blood gas test performed after weaning from cardiopulmonary bypass (CPB) showed compensated lactic metabolic acidosis (Table 1).
Table 1: Arterial blood gas analysis performed after weaning from CPB, showing compensated lactic metabolic acidosis with normocapnia under hyperventilation and a standard bicarbonate deficit of -3.6 mmol/L.
| Parameters | Value | Reference range |
| Lactate | 4,5 mmol/L | 0,5-2,2 mmol/L |
| pH | 7,36 | 7,35-7,45 |
| HCO2 (Bicarbonate) | 21,5 mEq/L | 22-28 mEq/L |
| Base Excess (BE) | -4 | -2 to +2 |
| PaCO2 | 38 mmHg | 35-45 mmHg |
| Hematocrit (Ht) | 20% | 37-47% (female) |
| PaO2 | 134 mmHg | 75-100 mmHg |
| Sodium (Na+) | 131 mmol/L | 135-145 mmol/L |
| Potassium (K+) | 2,4 mmol/L | 3,5- 5,0 mmol/L |
| Calcium (Ca2+) | 0,88 mmol/L | 1,12-1,32 mmol/L |
| Blood Glucose | 250 mg/dL | 70-100 mg/dL (fastimg) |
Two hours post-operatively, the patient received 2 units of packed red blood cells, with correction of potassium levels and calcium supplementation. A blood gas test revealed compensated lactic metabolic acidosis (Table 2).
Table 2: Arterial blood gas analysis performed 2 hours after weaning from CPB, showing compensated lactic metabolic acidosis with hypocapnia under hyperventilation and a standard bicarbonate deficit of -5 mmol/L.
| Parameters | Value | Reference range |
| Lactate | 4,5 mmol/L | 0,5-2,2 mmol/L |
| pH | 7,37 | 7,35-7,45 |
| HCO2 (Bicarbonate) | 21 mEq/L | 22-28 mEq/L |
| Base Excess (BE) | -5 | -2 to +2 |
| PaCO2 | 34 mmHg | 35-45 mmHg |
| Hematocrit (Ht) | 25% | 37-47% (female) |
| PaO2 | 132 mmHg | 75-100 mmHg |
| Sodium (Na+) | 131 mmol/L | 135-145 mmol/L |
| Potassium (K+) | 3,5 mmol/L | 3,5- 5,0 mmol/L |
| Calcium (Ca2+) | 0,99 mmol/L | 1,12-1,32 mmol/L |
| Blood Glucose | 230 mg/dL | 70-100 mg/dL (fastimg) |
Repeated TEE showed reassuring results regarding the function of the mechanical valve. in 2D and Doppler (Figure 10,11). Then, the patient was successfully extubated on day 2, adjusted-dose heparin (HBP) was administered with stable vitals. ACT was maintained within the therapeutic range, and the patient remained asymptomatic.
Figure 10: Postoperative continuous Doppler TEE on the mechanical mitral valve 1 hour after weaning from CPB, showing a mean gradient of approximately 4 mm Hg, with a mitral valve area of around 22 mmHg.
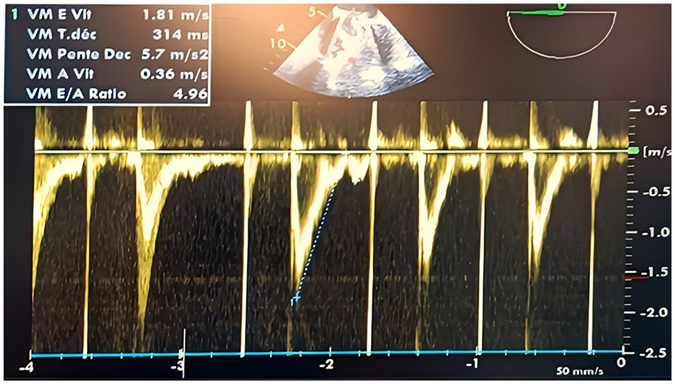
Figure 11: Postoperative pulsed Doppler TEE after weaning from CPB on the mechanical mitral valve showing an antegrade mitral flow velocity of the E wave at 2 m/s (greaterthan 1.5 m/s) and an E/A ratio of 4.96, indicating elevated filling pressures in the absence of an A wave due to atrial fibrillation.
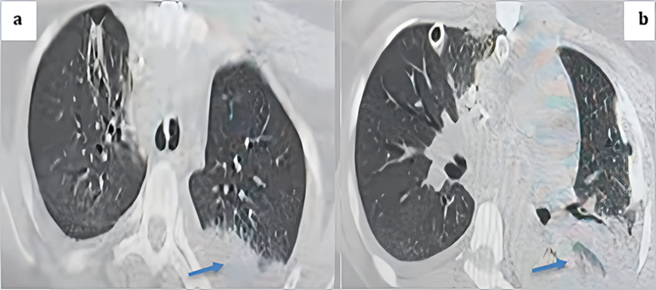
The following day, the patient exhibited signs of instability, including low blood pressure, reduced urine output, fever, increased heart rate, and higher oxygen requirements. A transthoracic echocardiogram (TTE) revealed no abnormalities with the heart valves or pericardial effusion. Blood cultures were collected, and antibiotics were initiated for pneumonia. Both chest X-ray and CT scan confirmed the diagnosis of pneumonia (Figure 12). Despite intensive treatment efforts, the patient developed refractory septic shock originating from the lungs and unfortunately passed away on postoperative day 4.
Figure 12: Thoracic CT scan in the parenchymal window with axial cuts showing a posterior basal left alveolar consolidation syndrome consistent with pneumonia, given the clinical context of the patient (blue arrows).
Discussion
Thrombosis is a significant complication of mechanical mitral valve replacement, caused by factors such as blood flow disturbances, valve design, inadequate anticoagulation, atrial fibrillation, and patient predispositions. [5,6] Effective management requires understanding the condition’s etiology, pathophysiology, and individual risk factors. Thrombosis can lead to obstructive dysfunction, embolism, and symptoms ranging from cardiac issues to strokes. [7] Diagnosing it can be challenging, particularly when distinguishing from conditions like infective endocarditis. Early diagnosis and interdisciplinary management, especially in stroke cases, are crucial. [8,9] While Transthoracic or transesophageal echocardiography is key for diagnosis, clear treatment guidelines are lacking, highlighting the need for further research [10]. In intensive care settings, hemodynamic data guide urgent management in patients with hemodynamic instability. For those with systolic blood pressure >100 mmHg and mean arterial pressure >65 mmHg, but with a >10 mmHg drops from baseline or hypotension, intravenous fluid resuscitation is appropriate, considering renal function. Angiography via femoral vein access and cardiac output measurement can help assess mitral stenosis severity and treatment response. Rapid management includes diagnosing the cause of instability, initiating inotropes like dobutamine, invasive blood pressure monitoring, and balanced transfusion of packed red blood cells with fresh frozen plasma. Hemodynamically unstable patients require a careful approach to fluid management, vasodilation, and analgesia which was the case for our patient. [11] Early anticoagulation and opiates may suffice, but if ineffective, urgent surgical intervention is required. Thrombolytic therapy can be used as a bridge to surgery in complex cases, like thrombosed mitral and tricuspid prosthetic valve dysfunction, where it temporarily stabilizes the patient for surgical intervention. Respiratory instability in this context often results from reduced left ventricular ejection fraction, causing pulmonary congestion, edema, and pleural effusions. Severe cases may lead to cardiogenic shock or acute respiratory distress syndrome. Mechanical ventilation can help mitigate complications by reducing left ventricular workload. Inotropes are controversial due to their potential to increase transmitral pressure gradients and complications [2, 12].
A rapid diagnosis, high-flow oxygen therapy, and the maintenance of dobutamine and norepinephrine were essential to stabilize the patient after the failure of thrombolysis, despite a large thrombus and hemodynamic instability. This approach provided valuable time to prepare the patient for thrombectomy which aligns with the data in the literature [13,14]. Conversely, in asymptomatic patients with stable hemodynamics and small thrombus sizes, thrombolytic therapy and anticoagulation may be considered [14]. Hemodynamic compromise, however, warrants emergency heart valve prosthesis replacement [15]. Patient selection is crucial in determining whether surgery or thrombolytic therapy is the best approach for mechanical valve thrombosis, considering factors like thrombus size, mobility, embolic events, and clinical status. Tissue plasminogen activator (tPA) is commonly used for thrombolysis, with streptokinase as a cost-effective alternative but a higher risk of hemorrhage, [16] In our case, tenecteplase was used despite its application in mechanical valve thrombosis being still debated and remaining off-label (not officially approved). However, it has been employed in specific contexts due to its simplicity of administration and thrombolytic efficacy. The indications and doses are derived from case reports and limited series, often guided by a risk-benefit assessment in emergency situations. [12][16] In chronic prosthetic heart valve recipients, if hemorrhage is excluded, fibrinolytics can be administered immediately due to the high pretest probability of thrombosis. Thrombolytics are first-line treatment for hemodynamically unstable patients. [16] The resolution of dyspnea indicates successful clot lysis and restored valve patency. Generally, management decisions are based on thrombus characteristics, time since occlusion, patient condition, and hemodynamic stability. thrombolysis is preferred for patients with hemodynamic instability and no left atrial clot, while surgery is favored when clot presence is uncertain or confirmed. However, definitive guidelines are lacking, leaving decisions to clinical judgment, with a need for further research. [2] The rarity of MMVT complicates the development of standardized treatments, with current data largely retrospective [17].
When surgery isn't ideal, comorbidities, thrombophilia, immunosuppression, and obesity complicate management. Excessive anticoagulation risks are minimal but add complexity. Further research is needed to develop clear guidelines and optimize patient-specific treatment strategies [5]. In provider-dependent centers, advanced evaluation tools and predictive models improve MMVT management, incorporating therapies like aspirin, acenocoumarol, clopidogrel, and fibrinolysis. Transesophageal echocardiography (TEE) enhances safety, especially for low-risk patients eligible for surgery or thrombolytic therapy within 24 hours. A multidimensional score assessing thrombus size, stenosis, and embolic risk supports personalized treatment planning. Multidisciplinary collaboration, adherence to guidelines, and effective communication ensure optimal care and minimize complications [5]. It is this multidisciplinary approach that was adopted during the care pathway of our patient, where TEE was very useful in guiding therapeutic decisions and evaluating their effectiveness, as clearly demonstrated in our clinical case. Medical management focuses on symptom relief, restoring valve function, and preventing clot progression, with anticoagulants and thrombolytics started promptly. Warfarin remains a key treatment, requiring careful monitoring for efficacy [5]. Thrombolytic therapy is a key treatment option for acute mechanical mitral valve thrombosis (AMMVT), especially in life-threatening cases or when surgery is not feasible. Agents like tissue plasminogen activator (tPA), streptokinase, or recombinant plasminogen activators are often first-line treatments. Success rates for thrombolysis in valve mechanical anomalies (VMA) reach up to 70%, although precise candidate criteria remain underdefined [18]. Factors like age, valve condition, and renal function help guide therapy. Thrombolytic therapy is most effective within 5-10 days of symptom onset and is often used when symptoms appear acutely.
Thrombolytic therapy is a recognized treatment for mechanical mitral valve thrombosis (MMVT), particularly in high-risk surgical patients or those with limited resources. It involves weight-based dosing and close monitoring for side effects like hemorrhage. Adjuvant therapies, including anticoagulants and measures to optimize cardiac function, are often used alongside thrombolysis. [18] The efficacy of systemic thrombolysis during the early period must be approached with great vigilance and sensitivity in emergency situations complicated by hemodynamic instability due to a thrombus on multiple mechanical valve discs. The immediate recovery of clinical signs and hemodynamic functions is rare; it is often progressive over time require careful monitoring for systemic and pulmonary thromboembolism through physical exams, imaging, and lab tests, with surgical intervention as an option for failed thrombolysis. The occurrence of systemic thromboembolic complications is not uncommon. However, the utility of the combination of tissue plasminogen activator and acenocoumarol remains highly debated in the literature, weighing the associated hemorrhagic risk. [18] Low-dose thrombolytic therapy has shown effectiveness and safety, especially in cases of bileaflet mechanical mitral valve obstruction. If initial thrombolysis fails, repeat therapy may be attempted, and serial imaging can guide decisions on elective surgical intervention. [19] However, the short- and long-term outcomes of both surgical revision and thrombolytic therapy remain suboptimal, emphasizing the need for further research and refinement of adjunctive therapies to improve outcomes and reduce mortality. [13]
Patients with severe mitral stenosis or contraindications for anticoagulation are at high risk, requiring early intervention to prevent myocardial injury and complications. Immediate surgery is often necessary, as delayed treatment carries high mortality [2]. Prosthetic valve thrombosis poses significant surgical challenges, with emerging endovascular techniques, such as transesophageal echocardiography-guided percutaneous thrombectomy, showing promise but limited by certain thrombus types. If unsuccessful, options include emergency balloon mitral valvotomy or surgical thrombus resection [19]. In specialized centers, emergency thrombectomy or thrombolytic therapy minimizes complications. Treatment for emboli or ischemia may involve thrombolytics, valve replacement, or combined surgical approaches. Ongoing reassessment ensures stabilization and timely definitive treatment [18]. Surgery for mechanical mitral valve thrombosis (MMVT) can be conventional or alternative, sometimes involving cardiopulmonary bypass or atrial pacing, especially when thrombolysis is contraindicated by a fresh thrombus or stuck device. [20-22] Indications for surgery in MMVT include three key scenarios: (a) Hemodynamic instability: Patients with severe heart failure, mitral stenosis, or regurgitation resistant to treatment require urgent surgery, such as commissurotomy or valve replacement. (b) Minor or no hemorrhagic complications: Surgery is preferred for patients with no significant bleeding or manageable minor hemorrhagic issues. (c) Anticoagulation contraindications or failure: Patients intolerant to anticoagulants or with systemic emboli despite adequate anticoagulation need surgical intervention, prosthetic valve thrombosis with heart failure or sudden hemodynamic instability, such as massive pulmonary embolism in bi-leaflet disc valve thrombosis, is an absolute indication for immediate surgery. [12] [23] If fibrinolytic therapy fails, surgery remains the definitive treatment. Treatment options include thrombectomy or valve replacement under general anesthesia and cardiopulmonary bypass, the heart is arrested and the mitral valve accessed via trans-septal or left atriotomy this is how our patient was managed. [2] In acute total thrombosis, thrombectomy is the primary intervention, using closed or open techniques. [20-23] Surgical management of mechanical mitral valve thrombosis (MMVT) typically involves cardiopulmonary bypass (CPB), though it carries risks like bleeding and hypocoagulability, advanced techniques may be used to manage infections or coagulability issues. Thrombectomy aims for complete thrombus removal, while severe valve damage may require replacement with a new prosthetic valve, which is quicker and less prone to blood loss. Left atrial appendage amputation is recommended for atrial fibrillation. [10] [20] [23] In some cases, emergency thoracotomy without CPB is successfully performed. Prompt intervention is essential in cases of infective endocarditis, severe hemodynamic compromise, or thrombolytic therapy failure. [2] [5] Postoperative cares often include high-dose anticoagulation, but this increases bleeding risks. Postoperative complications like heart failure, thrombosis, or leakage demand timely management, including anticoagulants, antibiotics, or further surgeries. [6,7] [13] The transaortic or atrial approach may be used, with the transaortic approach offering faster visualization. The aortic approach involves arterial cannulation, heparinization to an activated clotting time of 250 seconds, defibrillation, and retrograde cardioplegia injection. [19]
The management and prevention of mechanical mitral valve thrombosis (MMVT) risk in young individuals is a delicate balance between preventing thromboembolic events through high INR targets and the risk of excessive blood loss during emergency surgery. Choosing the appropriate prosthesis is complex, and the guidance of an experienced surgeon is often crucial. They need long-term anticoagulation and regular monitoring. Though rare, prosthetic valve thrombosis is a life-threatening complication requiring prompt cardiac evaluation to prevent severe outcomes like acute heart failure. Early detection and treatment are critical. [24,25] Acenocoumarol, a vitamin K antagonist, is essential for preventing thrombotic complications in mechanical heart valves by inhibiting vitamin K-dependent clotting factors (II, VII, IX, X, and proteins C and S). Maintaining therapeutic PT-INR levels (typically 2–3) through regular monitoring is crucial, with anticoagulation often initiated on the first postoperative day, it is this anticoagulant that was prescribed to our patient after her first valve replacement. At the same time, all other vitamin K antagonist have the marketing authorization in this indication, such as warfarin, or phenprocoumon, which is less commonly used but still available in some European countries. They require regular monitoring and careful management of the INR to adjust the dose. For tricuspid valve replacements, low-dose aspirin (≤100 mg/day) is sometimes added to reduce thromboembolism risk. [24] Effective management requires balancing anticoagulation to avoid bleeding or thromboembolic complications, with frequent monitoring and dose adjustments, especially during therapy initiation due to individual variability. [24,25] Despite proper monitoring, patient variability can lead to under- or overtreatment, with valve thrombosis still occurring due to factors like valve type, atrial fibrillation, or low cardiac output. [26] Addressing modifiable risk factors and focusing on primary prevention are essential. Maintaining target INR requires adherence to treatment, diet, medications, and health status. Education and self-management training improve adherence and outcomes, as therapeutic range time is critical. Enhancing adherence, particularly with direct oral anticoagulants, offers an underutilized opportunity for better management. [26] Although novel anticoagulants are under investigation, their role in managing mechanical mitral valve thrombosis (MMVT) is unclear. Effective management relies on individualized treatment, patient education, and adherence, with future trials aimed at refining therapeutic strategies [27].
Conclusion
Mechanical mitral valve thrombosis (MMVT) poses significant challenges, especially in young patients without typical risk factors. Management complexities include diagnosing valve obstruction in unstable patients and addressing silent thrombosis in anticoagulated individuals. Treatment strategies-anticoagulation, thrombolysis, or surgery-require individualized assessment of risks and benefits. A case of a 22-year-old with MMVT and hemodynamic and respiratory instability illustrates these complexities. Initial thrombolytic therapy stabilized the patient for surgery, which was successful. Postoperative care included transitioning anticoagulation from acenocoumarol to warfarin, highlighting the importance of timely intervention and strategic management. Surgical thrombectomy and thrombolysis remain effective, particularly in unstable patients, with perioperative care being critical. Key insights emphasize addressing postoperative complications, optimizing anticoagulation, and preventive strategies like early imaging and fiberoptic valve checks before discharge. While no definitive standard exists, surgery is preferred for critically ill patients, whereas thrombolytics are more suited for stable cases, albeit with a pulmonary embolism risk.
Declarations
Participants Consent
All participants (patient and her family) have given their explicit consent for the publication of personal data concerning themselves as part of this study. They understand that this data may include information that could identify them in the context of the research findings. They have been informed about the purpose of this publication, the type of data that will be disclosed, and the potential implications. They acknowledge that this information will be publicly accessible after publication. They confirm that their consent is voluntary and that they have the right to withdraw it at any time prior to publication.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving humans were approved by the ethics committee of Avicenne Military Hospital, chaired by Colonel Major Dr. Radouane Niamane, Professor of Higher Education at the Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech, Morocco, and Head of the Rheumatology Department at Avicenne Military Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participant provided their written informed consent to participate in this study.
Author Agreement and Contribution
All authors contributed to the production of this article. They also declare that they have read and approved the final version of this manuscript. S. Khallikane: Conceptualization, Investigation, Methodology, Writing-Original Draft. B. Bencharfa: Conceptualization, Investigation, Methodology, Writing-Original Draft. N. Atmani: Investigation, Validation, Writing-Review and Editing. A. Abdou: Investigation, Validation, Writing-Review and Editing. A. Belhadj: Investigation, Validation, Writing-Review and Editing. Y. Aissaoui: Assistance in Obtaining References, Supervision, and Editing.
Funding
The author(s) declare that no financial support was received from any organization for the submitted work.
Acknowledgments
The authors want to express their appreciation and gratitude to the following medical professionals involved in this investigation: members of Doctor ( Drs Aoub Belhadj, Abdelmajid Bouzerda, Hamid Jalal, Youssef Qamouss, Issam Serghini, Reda Mounir, Mehdi Nabil, Mehdi Didi, Hicham Kbiri, Ayoub Bouchama, Dahioui Ayoub, Bassam Bencharfa) the Perfusionists’ Team (El hilali Otmane )-for their cooperation and meritorical support; members of the Anesthesia Team (Aziz Akhiri, Atif Bouhadane, Jaafari Mohamed, Lhbib)-for their contribution in obtaining images and data for this study; operating room instrument technician team members ( El hilali Otmane)– for his cooperation and support and members of the Postoperative Care Team (Abderrazzak ech-chahed, Azeroual Hamza, aiabbir Mohamed)-for their invaluable help in collecting samples for this investigation.
Conflict of Interest
All authors declare the following: that they have no conflicts of interest. The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. And that there are no other relationships or activities that could appear to have influenced the submitted work.
References
- Gündüz S, Kalçık M, Gürsoy MO, Güner A, Özkan M. (2020). Diagnosis, Treatment & Management of Prosthetic Valve Thrombosis: The Key Considerations. Expert Rev Med Devices. 17(3):209-221.
Publisher | Google Scholor - Özkan M, Gündüz S, Güner A, Kalçık M, Gürsoy MO, et al. (2022). Thrombolysis or Surgery in Patients with Obstructive Mechanical Valve Thrombosis: The Multicenter HATTUSHA Study. J Am Coll Cardiol. 79(10):977-989.
Publisher | Google Scholor - Serban A, Gavan D, Pepine D, Dadarlat A, Tomoaia R, et al. (2024). Mechanical Valve Thrombosis: Current Management and Differences Between Guidelines. Trends Cardiovasc Med. 34(6):351-359.
Publisher | Google Scholor - Galusko V, Ionescu A, Edwards A, Sekar B, Wong K, et al. (2022). Management of Mitral Stenosis: A Systematic Review of Clinical Practice Guidelines and Recommendations. Eur Heart J Qual Care Clin Outcomes. 8(6):602-618.
Publisher | Google Scholor - Soria Jiménez CE, Papolos AI, Kenigsberg BB, Ben-Dor I, Satler LF, et al. (2023). Management of Mechanical Prosthetic Heart Valve Thrombosis: JACC Review Topic of the Week. J Am Coll Cardiol. 81(21):2115-2127.
Publisher | Google Scholor - Botsile E, Mwita JC. (2020). Incidence and Risk Factors for Thromboembolism and Major Bleeding in Patients with Mechanical Heart Valves: A Tertiary Hospital-Based Study in Botswana. Cardiovasc J Afr. 31(4):185-189.
Publisher | Google Scholor - Sharif Khan H, Ijaz Z, Ali M, Saif M, Ishaq U, et al. (2020). Clinical Outcomes of Mechanical Prosthetic Valve Thrombosis. Cureus. 12(6):e8760.
Publisher | Google Scholor - Truchart J, Plein D, Balthazar T, Droogmans S, Van Loo I, et al. (2024). Mechanical Valve Obstructive Thrombosis in An Asymptomatic Patient: A Gap in Current Recommendations. Acta Cardiol. 79(1):77-78.
Publisher | Google Scholor - Serban A, Dadarlat-Pop A, Achim A, Gavan D, Pepine D, et al. (2023). Diagnosis of Left-Sided Mechanical Prosthetic Valve Thrombosis: A Pictorial Review. J Pers Med. 13(6):967.
Publisher | Google Scholor - Parizher G, Ali A, Cremer PC. (2024). Evaluation and Management of Mechanical Heart Valve Dysfunction and Thrombosis. Curr Cardiol Rep. 26(7):747-755.
Publisher | Google Scholor - Pinsky MR, Cecconi M, Chew MS, De Backer D, Douglas I, et al. (2022). Effective Hemodynamic Monitoring. Crit Care. 26(1):294.
Publisher | Google Scholor - Ebrahimi P, Sattartabar B, Taheri H, Soltani P, Bahiraie P, et al. (2024). Mechanical Prosthetic Valve Thrombosis: A Literature Review of Treatment Strategies. Curr Probl Cardiol. 49(7):102628.
Publisher | Google Scholor - Tagliari F, Correia MG, Amorim GD, Colafranceschi AS, Pedroso JM, et al. (2022). Clinical Features and Survival Analysis of Patients after Mechanical Heart Valve Replacement, with an Emphasis on Prosthetic Valve Thrombosis. Arq Bras Cardiol. 119(5):734-744.
Publisher | Google Scholor - J. Kontos GJ Jr, Schaff HV, Orszulak TA, Puga FJ, Pluth JR, et al. (1989). Thrombotic Obstruction of Disc Valves: Clinical Recognition and Surgical Management. Ann Thorac Surg. 48(1):60-65.
Publisher | Google Scholor - Mackman N, Bergmeier W, Stouffer GA, Weitz JI. (2020). Therapeutic Strategies for Thrombosis: New Targets and Approaches. Nat Rev Drug Discov. 19(5):333-352.
Publisher | Google Scholor - Obied HY, Ibrahim MF, Latroche BS, Mroue MM. (2009). Successful Thrombolytic Therapy for Stuck Mitral Mechanical Valve. Saudi Med J. 30(7):964-966.
Publisher | Google Scholor - Druteikaitė, Kotryna & Bėrontaitė, Simona & Valterytė, Gintarė & Jarusevicius, Gediminas. (2023). Prosthetic Valve Thrombosis: Literature Review and Two Case Reports. Cor et Vasa. 65:90-99.
Publisher | Google Scholor - Bhatia, S., Jain, S., Sharma, V., Mansuri, Z., Patel, K., et al. (2020). Clinical Profile of Patients with Prosthetic Heart Valve Thrombosis Undergoing Fibrinolytic Therapy and NYHA Class as A Predictor of Outcome. International Journal of Cardiovascular Practice, 1(1).
Publisher | Google Scholor - Sadeghipour P, Salehi M, Kohansal E, Mozafarybazargany M, Parsaee M, et al. (2024). Ultraslow Low-Dose Thrombolytic Therapy in Patients with Acute Mechanical Prosthetic Heart Valve Thrombosis - A Prospective Cohort Study. Int J Cardiol. 416:132507.
Publisher | Google Scholor - Belostotskiĭ VE, Barbukhatti KO, Kurapeev IS, Novikov VK. (2004). Thrombectomy as a Method of Surgical Treatment of Thrombosis of a Mechanical Prosthesis of The Heart Valve (A Description of Three Cases). Vestn Khir Im I I Grek. 2004;163(3):75-78.
Publisher | Google Scholor - Carrier M, Pellerin M, Dagenais F, Perrault LP, Petitclerc R, et al. (1999). Videoassisted Thrombectomy of Mechanical Prosthetic Heart Valves. J Heart Valve Dis. 8(4):404-406.
Publisher | Google Scholor - Moscarelli M, Nasso G, Vite G, Speziale G. (2021). Surgical Treatment of Prosthetic Mechanical Valve Thrombosis with The Aid of High-Flow CO2. Eur J Cardiothorac Surg. 59(4):908-910.
Publisher | Google Scholor - Pomerantzeff, P. M. A., Steffen, S. P., de Almeida Brandão, C. M., Lapenna, G. A., Tarasoutchi, F., et al. (2015). Surgical Thrombectomy of Mechanical Valve Thrombosis. The Journal of Heart Valve Disease, 24(6):780-784.
Publisher | Google Scholor - Dubey AK, Kalita J, Nizami MF, Kumar S, Misra UK. (2024). Stability of Anticoagulation Following Acenocoumarin in Stroke Patients: Role of Pharmacogenomics and Acquired Factors. Ann Indian Acad Neurol. 27(3):274-281.
Publisher | Google Scholor - Aikins J, Koomson A, Ladele M, Al-Nusair L, Ahmed A, et al. (2020). Anticoagulation and Antiplatelet Therapy in Patients with Prosthetic Heart Valves. J Card Surg. 35(12):3521-3529.
Publisher | Google Scholor - Johansson I, Benz AP, Kovalova T, Balasubramanian K, Fukakusa B, et al. (2024). Outcomes of Patients with a Mechanical Heart Valve and Poor Anticoagulation Control on Warfarin. Thromb Haemost. 124(7):613-624.
Publisher | Google Scholor - Heestermans M, Poenou G, Hamzeh-Cognasse H, Cognasse F, Bertoletti L. (2022). Anticoagulants: A Short History, Their Mechanism of Action, Pharmacology, and Indications. Cells. 11(20):3214.
Publisher | Google Scholor