Research Article
Limited Body MRI for Plasma Cell Disorder
1Consultant Musculoskeletal Radiologist, Warrington & Halton Teaching Hospitals NHS Foundation Trust, Lovely Lane, Warrington, Cheshire WA5 1QG, United Kingdom.
2Consultant Gastrointestinal and Genitourinary Radiologist, Warrington & Halton Teaching Hospitals NHS Foundation Trust, Lovely Lane, Warrington, Cheshire WA5 1QG, United Kingdom.
*Corresponding Author: Akash Ganguly, Consultant Musculoskeletal Radiologist, Warrington & Halton Teaching Hospitals NHS Foundation Trust, Lovely Lane, Warrington, Cheshire WA5 1QG, United Kingdom.
Citation: Ganguly A., Anosike C. (2024). Limited Body MRI for Plasma Cell Disorder, Journal of Hematology Research and Blood Disorders, BioRes Scientia Publishers. 1(1):1-7. DOI: 10.59657/jhrbd.brs.24. 001
Copyright: © 2024 Akash Ganguly, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: May 07, 2024 | Accepted: May 22, 2024 | Published: May 31, 2024
Abstract
Background: New innovations in medicine are irrelevant if they are not widely available. Whole-body (WB) MRI is the reference standard for multiple myeloma imaging according to multiple international guidelines. Traditional MRI WB studies take 30 to 90 minutes, making it difficult to incorporate these amongst routine examinations and can be a challenge for the sick patients. We offer a hybrid protocol with MRI of axial skeleton with supplementary radiographs to maximise diagnostic information.
Aim: Compare our performance against published data in literature.
Materials and Method: MRI was performed on a 3T MRI scanner covering the spine, pelvis and proximal femora. Scan time was routinely under 15 minutes.
Results: 98 patients with plasma cell disorders (33 multiple myeloma, 58 MGUS, 2 smouldering myeloma, 3 plasma cell leukaemia and 2 plasmacytoma) had the MRI. 26/33 patients with multiple myeloma (79%) and all patients with PCL (100%) had an abnormal MRI. MRI showed the full range of marrow replacement comparable with published figures.
Conclusion: While WB MRI with diffusion and contrast remain the gold standard, a limited MRI is well suited for most radiology departments that cannot offer WB-MRI, and can be easily available to all patients.
Keywords: myeloma; MRI; plasma cell disorder
Introduction
Multiple myeloma is a plasma cell dyscrasia, characterized by a proliferation and accumulation of monoclonal plasma cells, predominantly affecting patients above 40, with a median age of 66 years at diagnosis [1]. With an ever-increasing incidence rate, up by 15% in the last decade, there are around 5,800 new myeloma cases in the UK every year, accounting for 2% of all new cancer cases (2015-17) [2]. New innovations in medicine are irrelevant if they are not widely available for use. NICE (National Institute for Clinical Excellence) recommends that imaging should be offered to all patients with plasma cell disorder suspected to be myeloma [3]. Traditionally, plain radiographs were used as the standard for assessing bone lesions. However, MRI offers excellent soft tissue contrast, along with the ability to demonstrate marrow involvement and image the whole marrow compartment with or without bone destruction. This has led to the adoption of whole-body MRI as the reference standard for imaging patients with myeloma by multiple international guidelines including the IMWG (International Myeloma Working Group) [3-6].
This has the potential of increasing the workload of the already stretched MRI service delivery infrastructure for most General Hospitals and diagnostic centres, and polarisation of the availability of whole-body MRI imaging to large Teaching Hospitals and Oncology centres. The quoted time range of 30 to 90 minutes (average 45 minutes) for a WB MR imaging protocol (excluding time in moving patients), together with often elderly or ill patient being scanned, adds to the challenge. This has resulted in lack of wide acceptance of this superior imaging option, with potentially significant impact on management of patients with plasma cell disorder. At our hospital we offer a hybrid imaging protocol with MRI of axial skeleton, namely spine, pelvis and proximal femur (limited WB-MRI) with additional limited radiographs of chest, skull and humerus to provide maximum diagnostic information. Our limited WB -MRI examination takes less than 15 minutes.
Materials and Methods
The aim of our study was to assess the diagnostic efficacy of the limited WB MRI examination. A retrospective study was designed to review all limited MRI performed for patients with suspected plasma cell disorder at our department, over a 19-month period, from April 2019 to October 2020. Our MRI protocol (detailed below) is based on previously published marrow screening MRI protocol covering the axial skeleton [7]. Ethical approval was waived in view of the retrospective nature of the data collection. Our inclusion criteria were all patients with suspected plasma cell disorder who underwent a limited WB- MRI. Exclusion criteria was whole body MRI performed for any other clinical indication.
MRI Imaging protocol
MRI is well recognized for marrow screening. Our protocol includes sagittal T1 weighted TSE and sagittal STIR images of the cervical and thoracic spine including sternum anteriorly and, coronal T1 weighted TSE and coronal STIR images of the lumbosacral spine, including the pelvis and both proximal femora (Table 1). All of our limited WB- MRI for myeloma patients were performed on a Philips Ingenia 3T scanner. Our average scan time was under 15 minutes routinely (acquisition time under 11 minutes).
Table 1: MRI protocol.
| Parameter | T1 TSE SAG | STIR TSE SAG | T1 TSE COR | STIR TSE COR |
| Coverage | Base of skull-L1 | Base of skull-L1 | T12-Prox/mid femur | T12-Prox/mid femur |
| TE | 16 | 60 | 20 | 70 |
| TR | 400-600 | 3400-5000 | 500-700 | 3000-6000 |
| TI | - | 210 | - | 260 |
| FOV | 300x500 | 300x500 | 480x380 | 480x380 |
| Slice thickness | 5 | 5 | 6 | 6 |
| Slice gap | 2.5 | 2.5 | 2 | 2.5 |
| Acquisition voxel | 0.95x1.25 | 0.8x1 | 0.73x0.81 | 1.5x1.5 |
| Acquisition matrix | 316x399 | 376x449 | 656x464 | 300x237 |
| Time | 02:32 | 02:00 | 03:03 | 02:37 |
| Coils | dStream - Base, posterior, anterior, and neck/neck top coil | |||
Supplementary x-rays
The supplementary x-rays performed include skull, chest, humeri and distal femora.
Results
We started our hybrid protocol of MRI limited body imaging and supplementary x-rays in April 2019. Between April 2019 to October 2020, we imaged 98 patients with plasma cell disorders, including 33 patients with multiple myeloma, 58 MGUS (monoclonal gammopathy of undetermined significance), 2 smouldering myeloma, 3 plasma cell leukaemia (PCL) and 2 patients with plasmacytoma. There were 42 female and 56 male patients. The female patients ranged from 45 to 89 years and the male patients ranged from 36 to 87 years.
Twenty-six out of total 33 patients with multiple myeloma (79%) and all three patients with PCL (100%) had an abnormal MRI. MRI showed the full range of marrow replacement from focal to variegated/ ‘salt and pepper’ appearance and diffuse change (Figures 1 to 4). Ten out of 33 showed multiple lesions, 9/33 showed diffuse marrow change, 4/33 showed variegated marrow changes, 3/33 showed small focal change and 7 were normal. These findings are comparable with published figures which quote a normal MRI in up to 28% of patients with myeloma [8,9]. Two of three patients with PCL showed diffuse marrow change, 1/3 showed multiple lesions. The patients with smouldering myeloma and plasmacytoma had normal MRI. Four out of 58 patients with MGUS showed changes on MRI; 2 multiple lesions and 2 focal areas of marrow signal change. One of 33 patients with myeloma had a normal MRI but a skull lesion.
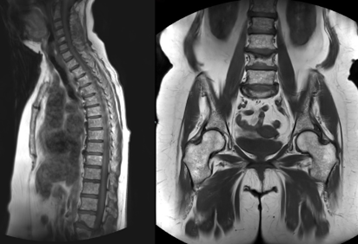
Figure 1: Salt and pepper appearance: Sagittal T1 weighted (a) and coronal T1 weighted (b) MR images of the spine, pelvis and proximal femora, of a 75-year-old lady with Myeloma, showing multiple tiny low signal lesions in keeping with ‘salt and pepper’ appearance.
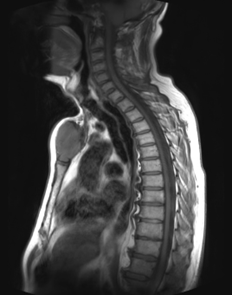
Figure 2: Plasmacytoma without marrow involvement: Sagittal T1 weighted MR image of the cervicothoracic spine of a 65-year-old man with a solitary Plasmacytoma without marrow involvement, showing an expansile lesion in the manubrium sterni.
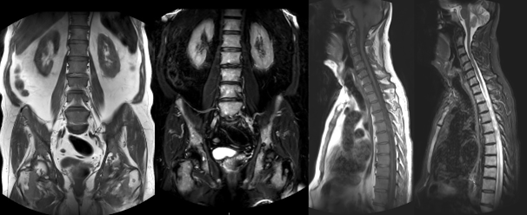
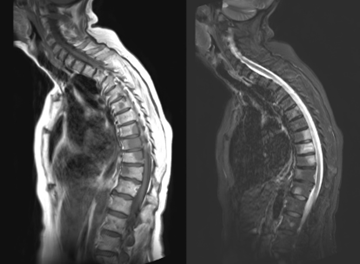
Figure 3: Diffuse marrow replacement: Coronal T1 weighted (a), coronal STIR weighted (b), sagittal T1 weighted (c) and sagittal STIR weighted (d) images of the spine, pelvis and proximal femora, in a 72-year-old man with Myeloma, showing diffuse marrow replacement with low signal on T1 weighted and high signal on STIR weighted images.
Figure 4: Multifocal lesions: Sagittal T1 weighted (a) and STIR weighted (b) images of the cervicothoracic spine of a 76-year-old lady with Myeloma, showing multiple discrete lesions involving several vertebrae.
Discussion
Multiple myeloma is a plasma cell dyscrasia, characterized by a proliferation and accumulation of monoclonal plasma cells. The disease evolves from an asymptomatic premalignant stage, MGUS, over smouldering multiple myeloma, to symptomatic myeloma with end-organ damage, such as hypercalcemia, renal impairment, anaemia and bone disease [8,10]. With an ever-increasing incidence rate, up by 15% in the last decade, there are around 5,800 new myeloma cases in the UK every year, accounting for 2% of all new cancer cases (2015-17) [2].
For decades the diagnosis of multiple myeloma required the presence of end-organ damage namely raised calcium level, renal dysfunction, anaemia, and bone lesions (CRAB features). Conventional radiographs were the gold standard for detecting bone lesions in myeloma based on the 2011 IMWG consensus guidelines. However, the sensitivity of skeletal survey was low as it required 30-50% loss of bone mass (sometimes 50-70% in the spine) to be detectable on plain films [11,6]. Unlike plain x-rays which detects bone destruction, MRI detects marrow infiltration. The excellent soft tissue contrast offered by MRI, along with its ability to image the whole marrow compartment (in contrast to marrow biopsy), prior to compromise in the bone structure, led to the inclusion of whole-body MRI as the reference standard for imaging in myeloma, in several internal guidelines including, IMWG, NICE and British Society of Haematologists [3-5]. The revised IMWG criteria (2014) allow, three myeloma defining events (MDE), which includes ‘more than one focal lesion on MRI’, to be considered sufficient for a diagnosis of multiple myeloma, regardless of the CRAB features [4].
Five patterns of bone marrow infiltration are recognised in multiple myeloma on MRI. These include, normal marrow signal (28%), focal lesions, diffuse infiltration, salt-and-pepper infiltrate and combined focal and diffuse infiltration [12]. MGUS patients, on the other hand have normal MRI. The presence of diffuse marrow infiltration and presence (and number) of unequivocal focal lesions, are recognised prognostic indicators of progression from MGUS/smouldering myeloma to symptomatic myeloma. MGUS is common in the population and increases with age, with a 1% average risk of progression to symptomatic myeloma; however, the progression rate of smouldering myeloma in comparison is 10% per year [13]. Studies have shown that finding more than one focal lesion on MRI is associated with a 70-80% risk of progression to symptomatic disease within two years [14,15].
Although myeloma can be diagnosed using non-imaging criteria, MRI plays a pivotal role in the management of plasma cell disorders. The IMWG consensus statement now recommends that smouldering myeloma patients with one or more unequivocal focal lesions (>5mm) should be treated as symptomatic myeloma and, for those where equivocal focal lesions are found, MRI should be repeated in 3–6 months to check for progression [6,8].
In addition to identifying changes to the marrow itself, MRI can also provide quantitative information such as tissue perfusion and composition/cellularity on diffusion-weighted imaging (DWI) [8]. Scan times reported for WB -MRI in literature ranges from 30 to 50 minutes, (average of 45 minutes), to more comprehensive ones lasting 90 minutes [8,6,16]. However, these protocols are difficult to implement due to constraints of an already overburdened MRI service delivery infrastructure and can be difficult to implement in routine practice for, a lot of General Hospitals in the UK with resource limitations and ever-increasing demand for scanner-time from competing specialties. Several departments with limited MRI scanner availability resort to CT or continue with x-ray skeletal survey for myeloma imaging, despite their reduced sensitivity in comparison to MRI. This leads to an increase in polarisation of availability of WB-MR imaging in large teaching hospitals and dedicated oncology centres. Another major disadvantage of long MRI scanning protocols is the challenge of imaging elderly and ill patients [8,17]. Longer time spent in the scanner often leads to deterioration of image quality and scan interruptions, more so in the elderly and infirm population.
Interestingly, limited magnetic resonance (MR) 'marrow screen' confined to the axial skeleton, similar to our protocol, is already in use without any significant loss of accuracy in staging patients for other malignancies where whole body scintigraphy was the reference standard [7].
Recognised protocols used include sagittal T1 and STIR weighted images of the spine covering the sternum anteriorly and T1 and STIR weighted images of the pelvis including proximal femora [7]. A recent American Society of Clinical Oncology consensus statement also highlights that approximately 90% of multiple myeloma lesions involve the axial skeleton [6]. Based on these, our protocol includes sagittal T1 weighted TSE and sagittal STIR images of the cervical and thoracic spine including sternum anteriorly and, coronal T1 weighted TSE and coronal STIR images of the lumbosacral spine, including the pelvis and both proximal femora.
The supplementary x-rays performed include skull, chest, humeri and distal femora. This was based on the fact that while it has been shown that, higher proportions of patients show focal lesions on MRI compared to plain radiographs in spine, pelvis, and sternum; the opposite is true for ribs, humeri, and femora with equal results for the skull [6,18]. Based on the above, and recognising that 10% of the patients may be misdiagnosed if only spine and pelvis is imaged [6,19].
We offer a limited body MRI to all our patients, a hybrid of MRI spine, pelvis and proximal femora supplemented by radiographs of the skull, chest, humeri and distal femora. Our MRI scanning time is less than 15 minutes which is very well tolerated by all our patients and has dove-tailed seamlessly with the rest of service demands. Our diagnostic rates are in line with published literature and guidelines with approximately 80% patients with myeloma and plasma cell leukaemia showing abnormalities on MRI.
Limitations
One of the limitations of this article is that it focuses mainly on the initial imaging for patients with plasma cell disorder. Myeloma is a disorder of marrow infiltration and bone destruction with the axial skeletal, in particular the spine and proximal long bones, being the most commonly involved [20]. It is well recognised that MRI of the spine and pelvis detects 90% of focal lesions and has been recommended as an alternative where full body MRI is not feasible [5,6].
Nevertheless, the authors recognise that when available, whole-body MRI with diffusion weighted and dynamic contrast enhanced images provide the most comprehensive information for patients with plasma cell disorder.
Conclusion
New innovations in medicine are only relevant if they can be universally rolled out and all hospitals and diagnostic centres can adopt it to benefit the patients they serve. MRI offers the best imaging modality for evaluation of plasma cell disorder, specifically myeloma, and should be available to every patient with suspected myeloma.
When WB- MRI is not feasible for any reason, offering a hybrid protocol covering the axial skeleton (namely spine, pelvis and proximal femora) on MRI and selected areas on X-ray provides critical information for patients with myeloma and other treatable plasma cell disorders, without creating significant strain on MR service provision. We recognise that WB MRI with DWI and dynamic contrast enhanced protocol is the gold standard. In addition, there is always scope to expand the protocol and incorporate other sequences, based on clinical requirement. However, considering the importance of MRI we believe, a shorter protocol, better tolerated by all age groups, including sick patients, could be a viable option for most radiology departments and diagnostic centres who are unable to offer the full WB MRI protocol to their patients.
Learning Points
- Whole Body MRI (WB-MRI) is the gold standard for imaging patients with myeloma.
- Limited body imaging allows short, well tolerated MRI protocol suitable for most busy radiology department, who cannot offer WB-MRI.
- Limited imaging protocols maintain sufficient diagnostic accuracy allowing management of patients with plasma cell disorder according to NICE/IMWG.
- MRI imaging should be available to all patients with plasma cell disorder.
Declarations
Funding
No funding was received by any of the authors.
Conflict of Interest
None of the authors have any conflict of interest to declare.
References
- Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, et al. (2003). Review of 1027 Patients with Newly Diagnosed Multiple Myeloma. Mayo Clin Proc. 78(1):21-33.
Publisher | Google Scholor - Cancer Research UK. (2014). Myeloma Incidence Statistics. Cancer Research UK.
Publisher | Google Scholor - NICE. (2016). Myeloma: Diagnosis and Management. NICE Guidelines.
Publisher | Google Scholor - Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, et al. (2015). International Myeloma Working Group Updated Criteria for The Diagnosis of Multiple Myeloma. The Lancet Oncology, 15(12):538-548.
Publisher | Google Scholor - Chantry A, Kazmi M, Barrington S, Goh V, Mulholland N, et al. (2017). Guidelines for the Use of Imaging in The Management of Patients with Myeloma. Br J Haematology, 178:380-393.
Publisher | Google Scholor - Dimopoulos MA, Hillengass J, Usmani S, Zamagni E, Lentzsch S, et al. (2015). Role Of Magnetic Resonance Imaging in The Management of Patients with Multiple Myeloma: A Consensus Statement. J Clin Oncol, 33:657-664.
Publisher | Google Scholor - Traill ZC, Talbot D, Golding S, Gleeson F V. (1999). Magnetic Resonance Imaging Versus Radionuclide Scintigraphy in Screening for Bone Metastases. Clin Radiol, 54(7):448-451.
Publisher | Google Scholor - Dutoit JC, Verstraete KL. (2016). MRI In Multiple Myeloma: A Pictorial Review of Diagnostic and Post-Treatment Findings. Insights into Imaging, 7:553-569.
Publisher | Google Scholor - Baur-Melnyk A, Buhmann S, Dürr HR, Reiser M. (2005). Role of MRI For the Diagnosis and Prognosis of Multiple Myeloma. Eur J Radiol, 55(1):56-63.
Publisher | Google Scholor - Kyle RA, Durie BGM, Rajkumar S V., Landgren O, Blade J, et al. (2010). Monoclonal Gammopathy of Undetermined Significance (MGUS) and Smoldering (Asymptomatic) Multiple Myeloma: IMWG Consensus Perspectives Risk Factors for Progression and Guidelines for Monitoring and Management. Leukemia, 24:1121-1127.
Publisher | Google Scholor - Hillengass J, Landgren O. (2013). Challenges and Opportunities of Novel Imaging Techniques in Monoclonal Plasma Cell Disorders: Imaging Early Myeloma. Leukemia and Lymphoma, 54(7):1355-1363.
Publisher | Google Scholor - Alyas F, Saifuddin A, Connell D. (2007). MR Imaging Evaluation of The Bone Marrow and Marrow Infiltrative Disorders of The Lumbar Spine. Magnetic Resonance Imaging Clinics of North America, 15(2):199-219.
Publisher | Google Scholor - Zingone A, Kuehl WM. (2011). Pathogenesis Of Monoclonal Gammopathy of Undetermined Significance and Progression to Multiple Myeloma. Semin Hematol, 48(1):4-12.
Publisher | Google Scholor - Hillengass J, Fechtner K, Weber MA, Bäuerle T, Ayyaz S, et al. (2010). Prognostic Significance of Focal Lesions in Whole-Body Magnetic Resonance Imaging in Patients with Asymptomatic Multiple Myeloma. J Clin Oncol, 28(9):1606-1610.
Publisher | Google Scholor - Kastritis E, Moulopoulos LA, Terpos E, Koutoulidis V, Dimopoulos MA. (2014). The Prognostic Importance of The Presence of More Than One Focal Lesion in Spine MRI of Patients with Asymptomatic (Smoldering) Multiple Myeloma. Leukemia, 28:2402-2403.
Publisher | Google Scholor - Messiou C, Kaiser M. (2015). Whole Body Diffusion Weighted MRI - A New View of Myeloma. British Journal of Haematology, 171:29-37.
Publisher | Google Scholor - Filho AGO, Carneiro BC, Pastore D, Silva IP, Yamashita SR, et al. (2019). Whole-Body Imaging of Multiple Myeloma: Diagnostic Criteria. Radiographics, 39(4):1077-1097.
Publisher | Google Scholor - Walker R, Barlogie B, Haessler J, Tricot G, Anaissie E, et al. (2007). Magnetic Resonance Imaging in Multiple Myeloma: Diagnostic and Clinical Implications. J Clin Oncol, 25:1121-1128.
Publisher | Google Scholor - Bäuerle T, Hillengass J, Fechtner K, Zechmann CM, Grenacher L, et al. (2009). Multiple Myeloma and Monoclonal Gammopathy of Undetermined Significance: Importance of Whole-Body Versus Spinal MR Imaging. Radiology, 252(2):477-485.
Publisher | Google Scholor - Shortt CP, Carty F, Murray JG. (2010). The Role of Whole-Body Imaging in The Diagnosis, Staging, And Follow-Up of Multiple Myeloma. Semin Musculoskelet Radiol, 14(1):37-46.
Publisher | Google Scholor