Case Report
Late Postpartum Pyrexia and Ascites Due to Peritoneal Tuberculosis
Post graduate institute of Medicine, University of Colombo, Sri Lanka.
*Corresponding Author: Sanjaya Walawe Nayaka,Post graduate institute of Medicine, University of Colombo, Sri Lanka.
Citation: Walawe Nayaka S. (2023). Late Postpartum Pyrexia and Ascites Due to Peritoneal Tuberculosis, Journal of Women Health Care & Gynecology, BioRes Scientia Publishers. 2(2); DOI: 10.59657/2993-0871.brs.23.009
Copyright: © 2023 Sanjaya Walawe Nayaka, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: July 28, 2023 | Accepted: August 18, 2023 | Published: August 22, 2023
Abstract
Postpartum pyrexia is a serious condition which associates with increased maternal morbidity and mortality. Even though it is mostly associated with genital tract infections, tuberculosis has to be considered in prolonged pyrexia without any diagnosis due to its increased prevalence and atypical ways of presentation. This is a case of peritoneal tuberculosis presented with post-partum ascites and fever.
Keywords: postpartum; maternal mortality; ascites
Introduction
Postpartum pyrexia and sepsis are two leading causes of preventable maternal morbidity and mortality in both developing countries and developed countries which contributes to 15% of all maternal deaths (75000 per year) worldwide [1,2]. Most of these infections are gained once the mother discharged from hospital, usually from the second day of puerperium. The World Health Organization (WHO), defines puerperal sepsis as “infection of the genital tract occurring at any time between the onset of the rupture of membranes or labor and the 42nd day postpartum in which fever and one or more of the following are present: pelvic pain, abnormal vaginal discharge, abnormal odor of discharge and delay in the rate of reduction of size of the uterus [2]. This has become one of the challenges obstetricians are faced for many decades due to its diagnostic dilemma. Caesarean section, episiotomy site, placental bed and cervical lacerations are at high risk of being infected following delivery [3]. Other infective causes which not related to genital tract are mastitis, venous thromboembolism, urinary tract infections, respiratory tract infections and cannula site infections. Tuberculosis (TB), human immune-deficiency virus (HIV), parasitic infections (like leishmaniosis), lymphomas and rheumatic carditis have to be considered in atypical cases with prolonged pyrexia. Very rarely fibroid complicating pregnancies may cause prolonged fever due to infarction and inflammation of the fibroid [4]. According to the onset of the fever, it can be divided into immediate (with in first 24 hours), mediate (between second to seventh day) and late (between second to sixth week) [5].
Following factors are known to increase the risk of postpartum infections.
- prolonged rupture of membranes more than 18 hours before delivery
- pre-existing vaginal infection or history of Group B Streptococcal infection
- wound hematomas
- retained products of conception or blood clots inside the uterine cavity
- low immune status due to drugs, chronic diabetes, co-existing malignancies
- anaemia
- obesity (specially in developed world)
Pyrexia of unknown origin (PUO) is defined as fever more than 38.3 0C for three weeks without any diagnosis in spite of investigating for one week [6]. Both infectious causes and non-infectious causes have to be considered when treating a postpartum woman with PUO.
TB remains as one of the leading diseases among infectious causes of PUO as it is a disease with high burden [7]. HIV, alcohol, smoking and diabetes contribute to increase the prevalence of the TB which twice in men compare to the women [8]. Prevalence of TB among pregnant women in America was reported as 7.1 per 100,000 hospitalizations [9]. Prevalence of miliary TB (MTB) is 1-2% of all TB cases and occurred due to wide spread dissemination of the Mycobacterium tuberculosis. Risk factors for MTB are female gender, poor socio-economic status, immunosuppressed state, extremes of age and alcoholism [10-12]. Anemia of the baby, preeclampsia, pneumonia, preterm labor, congenital infection and intra-uterine death are associated with delay in diagnosis and treatment [13-15]. Incidence of TB in Sri Lanka in 2021 was 63 cases per 100,000 people [16]. In peritoneal TB (PTB) bacilli commonly enters to the peritoneal cavity through haematogenous spread from pulmonary focus or rarely through the diseased bowel [17]. HIV infection, diabetes mellitus, treatment with anti-tumor necrosis factor (TNF) agents, ongoing peritoneal dialysis and hepatic cirrhosis are the risk factors for PTB [18]. In PTB, the patients present with PUO, abdominal distention, abdominal pain and ascites.
Case history
30-year-old lady who was a mother of 3-month-old, breast fed, vaginally delivered child presented with progressive abdominal fullness and intermittent fever for the last 2 months. She was apparently well up to fourth postpartum week until she notices gradually worsening abdominal fullness associated with intermittent fever, loss of appetite and loss of weight. She didn’t have any features suggestive of urinary tract, genital tract, respiratory tract or gastro intestinal tract infections. She denied any past contact history of TB. Her ovaries and uterus were said to be normal during antenatal ultrasonography. She didn’t have any significant past medical history even though she had undergone appendectomy five years ago. Her pregnancy and delivery were uncomplicated and her menstruation had not started. She denied any family history of malignancies.
On examination she was febrile (1010F), not pale, not dyspneic in supine position, not icteric and no ankle edema. She did not have cervical lymphadenopathy. There was no any abnormality detected in cardiovascular system, respiratory system and breast examination. There were no any signs suggestive of deep vein thrombosis. Her abdomen was symmetrically enlarged and there were no organomegaly. She had flank dullness and shifting dullness which suggestive of ascites. Her pelvic examination was normal. She had been treated with multiple antibiotics during last few weeks without any clinical improvement. Her investigation results are as follows.
Table 1: summary of the investigation results.
| Investigation | Results |
| White blood cells (WBC) | 8830/ul |
| Hemoglobin | 10.4g/dl |
| Platelet | 479000/ul |
| C-reactive protein | 223ng/ml |
| Blood urea, serum creatinine | Within normal range |
| Serum sodium, potassium and chloride | Normal |
| AST, ALT, ALP | Normal |
| Total protein | 7.3g/dl |
| Albumin | 3g/dl |
| Globulin | 4.3g/dl |
| Total and direct bilirubin | Normal |
| ESR | 80mm/first hour |
| CA 125 | 285 iu/ml |
| Dengue NS1 antigen | Negative |
| Dengue antibody | Negative |
| Blood picture | Reactive picture |
| Blood culture and urine culture | No growth |
| Ultra sound scan of the abdomen and pelvis | Gross ascites with normal abdomino-pelvic organs. |
| Computerized tomography scan | Gross ascites and small pleural effusion |
| Echo cardiogram | Normal |
| Peritoneal fluid aspiration results | |
| WBC | 1310/ml |
| Mononuclear cells | 10% |
| Polymorphonuclear cells | 90% |
| Glucose | 95mg/dl |
| Lactic dehydrogenas | 382u/l |
| Protein | 5.26g/dl |
| Culture | No bacterial growth/ TB culture results pending |
| TB PCR (polymerase chain reaction) | Results pending |
| Staining for acid fast bacilli (AFB) | Negative |
| Mantoux test done | Awaiting reading the results |
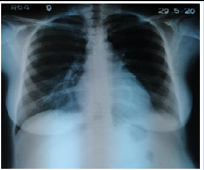
| Chest X-ray | Normal |
| Sputum for AFB | Negative |
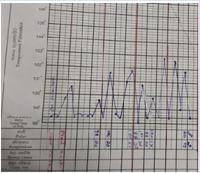
Figure 1: Temperature chart
Figure 2: Chest X-ray
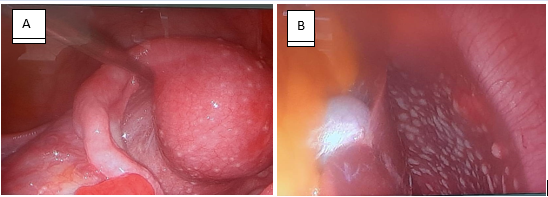
Multi-disciplinary team (MDT) meeting was arranged and decided to proceed with diagnostic laparoscopy as the possibility of peritoneal TB was high and results of confirmatory tests were pending while patient was suffering due to the symptoms. Diffuse, small (less than 1cm) whitish tubercles noted all over the parietal peritoneum, uterine surface, tubal surface, pouch of Douglas, bowel, liver surface and undersurface of the diaphragm during the diagnostic laparoscopy. Straw colored ascites was noted. Ovaries and other abdomino-pelvic organs didn’t demonstrate any features suggestive of malignancy. Multiple biopsies were taken from those lesions and sent for TB culture and histology.
These laparoscopy findings were discussed in detail during next MDT meeting and decided to treat as PTB and anti TB drug therapy was started. Her 3 months old baby was investigated by neonatology team for TB and found to be non-infected. Her fever settled after 48 hours of starting treatment and her all constitutional symptoms gradually settled. TB PCR was positive and histology confirmed the presence of granuloma formation with caseous necrosis which suggestive of TB. Her culture report came later as positive for Mycobacterium tuberculosis. She completed her treatment course and fully recovered from the disease.
Figure 3: Laparoscopic view of uterus (A) and liver (B) with TB infection.
Discussion
PTB accounts 4.9% of all extra pulmonary TB cases and it is the sixth commonest site to have extra pulmonary TB [19]. This case shows the importance of considering TB as a cause of PUO in postpartum women who are from endemic areas presented with nonspecific constitutional symptoms. Pregnancy and postpartum period up to 180 days had been identified as a unique risk factor for the activation of latent TB [20]. Downregulation of Th-1 cytokine and natural killer cell cytotoxicity and a reduction in interferon gamma production which taken place during pregnancy as a physiological change adversely affect the latent TB to reactivate in females compare to males. As most common symptoms of PTB are slowly progressing abdominal pain (50-100%) and abdominal distention (40-73%) patients seek treatments after having symptoms for months [17]. There may be other associated symptoms like fever (13-59%), night sweat (6%) and loss of weight (50-61%) may open the eyes of the patients to seek treatments. Even though the lungs are the primary focus of PTB, only one third of them show clinical or radiological signs and symptoms of it [21]. Thus, PTB becomes a diagnostic challenge to the physician. In this case patient may have had symptoms even before the delivery of the baby which may have masked by the gravid uterus and normal pregnancy symptoms.
Even though full blood count and other basic investigations not confirm the final diagnosis, performing liver, renal and cardiac function tests are important to exclude renal, hepatic and cardiac causes which can lead to ascites. Peritoneal fluid analysis plays a major role in diagnosing PTB. 68% of patients with PTB having lymphocytic predominant WBC containing (500-1500/mm3) ascites with protein contain >2.5g/dl [22]. All these features were present in this patient.
Peritoneal thickening (23-32%), lymphadenopathy (14-47%) and loculated or localized ascites (36-67%) are the common features in ultra sound scan and CT scan of a patient with PTB [23].
Sensitivity of Ziehl-Neelsen stain of the ascetic fluid is 0-6% while sensitivity of culture of ascetic fluid is16 to 58%. This can be improved by centrifuging a large sample [17]. Even though TB PCR from ascetic fluid is highly specific, it has low sensitivity. Adenosine deaminase level of >30 IU/L in peritoneal fluid is highly suggestive of PTB which having sensitivity closer to 100% and specificity more than 95% for diagnosing PTB [22].
Performing Mantoux test will not differentiate between latent TB and active TB even though it is supporting in diagnosis [22]. Even though positive culture is the gold standard of diagnosing the TB, disease can’t be excluded by a negative culture. Consumption of long time period for this directs the physicians to go ahead with additional methods like diagnostic laparoscopy which allows for the direct visualization of the peritoneal cavity, come to a diagnosis and obtain samples directly from the lesions [24]. There are three different characteristic laparoscopic appearances have been described in PTB (substantial peritoneal thickening with dense adhesions that may extend to adjacent organs, a thickened peritoneum with yellow-white tubercles and a thickened peritoneum without tubercles) [25].
Even after all these investigations, diagnosis may not be confirmed and trial of anti TB therapy in such occasion may help to come to a definitive diagnosis as patients symptoms start to improve after starting treatment in patients with PTB. High index of suspicion and proper investigations are the two most important things in reduce the maternal morbidity and mortality in postpartum PUO due to TB.
Conclusion
PTB should be considered as a differential diagnosis in women who present with postpartum PUO and abdominal distention, especially who live in TB endemic areas. Proper history, examination and appropriate investigations will reduce the delay in starting anti TB treatments. Diagnostic laparoscopy plays an important role in cases where the diagnosis is inconclusive. Trial of anti TB treatments in highly selected cases can be justifiable as some confirmatory tests take long time to give the results.
Declarations
Conflict of interest: No conflict of interest.
Funding: No funding
Consent: Informed written consent obtained from the patient.
References
- Loudon I. (2000). The cause and prevention of puerperal sepsis. JR Soc Med, 93:394-395.
Publisher | Google Scholor - Kainer F. (2006). Postpartum infection. Gynakol Prax, 30:201-207.
Publisher | Google Scholor - Drife J. (2001). Infection and maternal mortality. In: MacLean AB, Regan L, Carrington D, eds. Infection and Pregnancy. London:RCOG Press,:355-364
Publisher | Google Scholor - Lee WL, Chiu LM, Wang PH, Chao HT, Yuan CC, Ng HT. (1998). Fever of unknown origin in the puerperium. A case report. J Reprod Med, 43(2):149-152. PMID: 9513878.
Publisher | Google Scholor - Miller A. (2012). Review of the bacteriology of puerperal sepsis in resource-poor settings. Melbourne: Compass: Women’s and Children’s Health Knowledge Hub.
Publisher | Google Scholor - Harper MA. Post-partum pyrexia of unknown origin: case based learning; OBSTETRICS, GYNAECOLOGY AND REPRODUCTIVE MEDICINE 20:2,57.
Publisher | Google Scholor - Mert A, Arslan F, Kuyucu T, Koc EN, Ylmaz M, Turan D, Altn S, et al. (2017). Miliary tuberculosis: Epidemiologicaland clinical analysis of large-case series from moderate to low tuberculosis endemic Country. Medicine (Baltimore), 96(5):5875.
Publisher | Google Scholor - Collaborators GBDT. (2018). The global burden of tuberculosis: results from the Global Burden of Disease Study 2015. Lancet Infect Dis,18(3):261-284.
Publisher | Google Scholor - Dennis EM, Hao Y, Tamambang M, Roshan TN, Gatlin KJ, Bghigh H, Ogunyemi OT, et al. (2018). Tuberculosis during pregnancy in the United States: Racial/ethnic disparities in pregnancy complications and in-hospital death. PLoS One,13(3):0194836.
Publisher | Google Scholor - Webster AS, Shandera WX. (2014). The extrapulmonary dissemination of tuberculosis: A meta-analysis. Int J Mycobacteriol, 3(1):9-16.
Publisher | Google Scholor - Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR. (2009). Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis, 49(9):1350-1357.
Publisher | Google Scholor - Abad CLR, Razonable RR. (2018). Mycobacterium tuberculosis after solid organ transplantation: A review of more than 2000 cases. Clin Transplant, 32(6):13259.
Publisher | Google Scholor - El-Messidi A, Czuzoj-Shulman N, Spence AR, Abenhai HA. (2016). Medical and obstetric outcomes among pregnant women with tuberculosis: a population-based study of 7.8 million births. Am J Obstet Gynecol, 215(6):797.1-797.
Publisher | Google Scholor - Bates M, Ahmed Y, Kapata N, Maeurer M, Mwaba P, Zumla A. (2015). Perspectives on tuberculosis in pregnancy. Int J Infect Dis, 32:124-127.
Publisher | Google Scholor - Peng W, Yang J, Liu E. (2011). Analysis of 170 cases of congenital TB reported in the literature between 1946 and 2009. Pediatr Pulmonol, 46(12):1215-1224.
Publisher | Google Scholor - World Data Atlas. (2021). Sri Lanka - Incidence of tuberculosis.
Publisher | Google Scholor - Uygur-Bayramicli O, Dabak G, Dabak R. (2003). A clinicaldilemma: abdominal tuberculosis. World J Gastroenterol. 9:1098-1101.
Publisher | Google Scholor - Terras Alexandre A, et al. (2017). Peritoneal tuberculosis - A rare diagnosis. Rev Port Pneumol. http://dx.doi.org/10.1016/j.rppnen.2017.02.002
Publisher | Google Scholor - Peto HM, Pratt RH, Harrington TA, et al. (2009). Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis, 49:1350–1357.
Publisher | Google Scholor - Malhame I, Cormier M, Sugarman J, Schwartzman K. (2016). Latent Tuberculosis in Pregnancy: A Systematic Review. PLoS One,11(5):0154825.
Publisher | Google Scholor - Mimidis K, Ritis K, Kartalis G. (2005). Peritoneal tuberculosis. Ann Gas-troenterol, 18:325-329.
Publisher | Google Scholor - Sanai FM, Bzeizi KI. (2005). Systematic review: tuberculous peritonitis--presenting features, diagnostic strategies and treatment. Aliment Pharmacol Ther, 22:685–700.
Publisher | Google Scholor - Kedar RP, Shah PP, Shivde RS, et al. (1994). Sonographic findings in gastrointestinal and peritoneal tuberculosis. Clin Radiol, 49:24–29.
Publisher | Google Scholor - Kılıc¸ MÖ, Sa˘glam C. (2016). Evaluation of forty-nine patients with abdom-inal tuberculosis. J Clin Anal Med, 7:470-474.
Publisher | Google Scholor - Bhargava DK, Chopra P, Nijhawan S, et al. (1992). Peritoneal tuberculosis: laparoscopic patterns and its diagnostic accuracy. Am J Gastroenterol, 87:109–112.
Publisher | Google Scholor