Case Report
Investigation Of Fluoride Contents in Teeth Using a Fluoride Selective Electrode
Department of Chemical Technology, Technical sciences vocational school, Gazi University, Ankara, Turkey.
*Corresponding Author: Şükrü Kalayci,Department of Chemical Technology, Technical sciences vocational school, Gazi University, Ankara, Turkey.
Citation: Şükrü Kalayci. (2024). Investigation of Fluoride Contents in Teeth Using a Fluoride Selective Electrode, Dentistry and Oral Health Care, BioRes Scientia Publishers, 2(2):1-3. DOI: 10.59657/2993-0863.brs.24.026
Copyright: © 2024 Şükrü Kalaycı, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: December 06, 2023 | Accepted: January 27, 2024 | Published: January 08, 2024
Abstract
It is important to examine the amount of fluoride for dental health. A fluoride selective electrode was developed for the determination of fluoride amount. A fluoride selective electrode was prepared by using a fluoride-containing ionophore and slightly soluble sulfur salts in certain proportions. This electrode showed an analytical slope of approximately 30 mV for fluoride concentrations. Optimum conditions were determined by examining the factors affecting the sensitivity of this electrode. Fluoride analysis was performed on 4 different tooth samples taken from Gazi University faculty of dentistry. Fluoride amounts of the same samples were measured using an ion chromatography device. The results obtained were found to be compatible.
Keywords: teeth; fluoride; selective electrode; potentiometry
Introduction
Fluoride is an important element in maintaining dental health. Dental health deteriorates in lack or excess of fluoride. The change in the amount of fluoride is mostly due to the water and food consumed. It has been determined that the amount of fluoride changes as a result of environmental pollution of water and chemicals used in food production, and therefore dental health deteriorates [1-5]. Determining the amount of fluoride also becomes extremely important. Fluoride determination was made by many methods. These methods include FT-IR spectroscopy [6], Raman spectroscopy [7], ion chromatography [8] and ICP-OES methods [9]. Analyzes performed with these methods are expensive and have many interference effects. In this study, analysis of the amount of fluoride was performed using a fluoride selective electrode. Analytical performance values of the electrode were measured and its sensitivity was determined to be high. The amounts of fluoride in 4 different tooth samples were determined with a fluoride selective electrode.
Materials And Methods
Materials and Reagents
Calcium fluoride (Sigma), used as an ionophore, Ag(I)S and CuS were commercially obtained. Anion and cation solutions (Merck) were obtained from solid salts. All solutions were prepared using twice purified water.
Instruments
An external Ag/AgCl reference electrode with a Jenway 3040 pH/ion meter was used for potential measurement. Potential values were measured at room temperature.
Preparation of fluoride selective electrode
A certain amount of the salt or salt mixture of Ag(I)S, CuS and CaF2 (10–15 mg) was taken and pellets were made by holding first under a pressure of 1940 atm for 1min, then pressure was increased to 7760 atm by adding each time 1940 atm for 1min. The pellets of 7mmdiameter were sealed with epoxy resin (0.7 g epoxy and 0.9 ghardener). To obtain a good sealing the epoxy resin on the edge of the tubing had to wait for about 10 min and then the pellet was sealed. One day after the pellet was sealed a silver wire is connected. For this purpose, the inside of the glass tube was filled to about 1 cm with a mixture of 0.5 g graphite powder and epoxy resin. The electrode prepared had to wait for about 2 days so that the resin can get dry. The surface of the electrode is washed and then polished with a soft paper [10].
Preparation of teeth samples
Four dental samples were taken from the faculty of dentistry. Tooth samples were kept in 5ml TISAB II (Total Ionic Strength Adjustments Buffer) for 24 hours. The amount of fluoride passing into the solution was measured using an electrode.
Results and discussion
Sensitivity of fluoride electrode
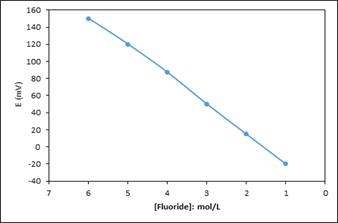
The sensitivity of electrode prepared for fluoride ion was measured. For this purpose, in the presence of 0.1 M NaNO3 solution, the fluoride concentration in the solution was increased by 10 times from 1x10 -6 M to 0.1 M fluoride concentration and the changes in the potential were recorded. At this point, the slope of the electrode was calculated as 30 ± 0.1 mV. Electrode sensitivity is given in figure 1.
Figure 1: Calibration chart of fluoride electrode.
Analytical performance of fluoride electrode
Samples containing known amounts of fluoride, which are necessary to determine the amount of fluoride that may be present in the tooth sample, were analysed with the fluoride selective electrode we prepared. Results were calculated as the mean of 4 experiments based on a 95% confidence interval. Analytical performance values of the electrode are given in Table 1.
Table 1: Analytical performance values of thiosulfate membrane electrode
| Amount of Fluoride added: µg/L | Measurement x ̅ ± ts / √N | Recovery, % |
| 10 | 9,1 ± 0,1 | 91 |
| 25 | 24,2 ± 0,3 | 97 |
| 50 | 48,5 ± 0,7 | 97 |
| 100 | 98,6 ± 4,5 | 99 |
(CI 95%, N=4  : Arithmetic mean, s: Standard deviation, t: Student’s t-test).
: Arithmetic mean, s: Standard deviation, t: Student’s t-test).
Determination of fluoride in teeth samples
Fluoride in teeth samples of 4 different patients was measured both with the fluoride selective electrode we prepared and with ion chromatography in the hospital laboratory. The results are given in table 2 as the average of 4 experiments, according to the 95% confidence interval.
Table 2: Comparison of the amount of fluoride in dental samples with both methods
| Teeth samples | Electrode with found [Fluoride]: µg/L | Ion Chromatography with found [Fluoride]: µg/L |
| 1 | 10 ± 2 | 9 |
| 2 | 35 ± 0,2 | 36 |
| 3 | 108 ± 3 | 117 |
| 4 | 203 ± 4 | 205 |
(CI 95%, N=4  : Arithmetic mean, s: Standard deviation, t: Student’s t-test).
: Arithmetic mean, s: Standard deviation, t: Student’s t-test).
Conclusion
It was measured that the fluoride selective electrode, using calcium fluoride as the ionophore and prepared from slightly soluble sulfur salts, was sensitive to fluoride concentration with a slope of approximately 30 mV. It was determined that the analytical performance of the electrode was high against synthetically added fluoride concentrations. Especially the % recovery rates of 97-99% confirm this. Then, 4 different tooth samples were kept in TISAB II solution for 24 hours and fluoride analyzes were measured both by electrode and ion chromatography. It was determined that the results were compatible with each other. Using fluoride electrodes in dental samples was found to be extremely practical and easy to analyze.
Conclusion
It was measured that the fluoride selective electrode, using calcium fluoride as the ionophore and prepared from slightly soluble sulfur salts, was sensitive to fluoride concentration with a slope of approximately 30 mV. It was determined that the analytical performance of the electrode was high against synthetically added fluoride concentrations. Especially the % recovery rates of 97-99% confirm this. Then, 4 different tooth samples were kept in TISAB II solution for 24 hours and fluoride analyzes were measured both by electrode and ion chromatography. It was determined that the results were compatible with each other. Using fluoride electrodes in dental samples was found to be extremely practical and easy to analyze.
Declaration
Acknowledgments
The authors would like to thank Gazi University, Department of Chemical Technology.
Conflict of Interest
The authors declare that they have no competing interests.
References
- Paul T.C. Harrison. (2005). “Fluoride in water: A UK perspective”. Journal of Fluorine Chemistry, 126(11-12):1448-1456.
Publisher | Google Scholor - Z. Mandinic, M. Curcic, B. Antonijevic, M. Carevic, J. Mandic, D. Djukic-Cosic, C.P. Lekic. (2010). “Fluoride in drinking water and dental fluorosis”. Science of The Total Environment, 408(17):3507-3512.
Publisher | Google Scholor - P. James, M. Harding, T. Beecher, D. Browne, M. Cronin, H. Guiney, D. O’Mullane, H. Whelton (2021). “Impact of Reducing Water Fluoride on Dental Caries and Fluorosis”. Journal of Dental Research, 100(5):507-514.
Publisher | Google Scholor - J. Opydo-Szymaczek, M. Ogińska, B. Wyrwas (2021). “Fluoride exposure and factors affecting dental caries in preschool children living in two areas with different natural levels of fluorides”. Journal of Trace Elements in Medicine and Biology, 65:126726-126731.
Publisher | Google Scholor - B. Sawangjang, S. Takizawa. (2023). “Re-evaluating fluoride intake from food and drinking water: Effect of boiling and fluoride adsorption on food”. Journal of Hazardous Materials, 443(Part A):130162:130167.
Publisher | Google Scholor - M. Porto, R. A. Saiani, K.L. A. Chan, S. G. Kazarian, R. F. Gerlach, L. Bachmann. (2010). “Organic and inorganic content of fluorotic rat incisors measured by FTIR spectroscopy”. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 77(1):59-63.
Publisher | Google Scholor - J. L. González-Solís, E. Martínez-Cano, Y. Magaña-López. (2015). “Early detection of dental fluorosis using Raman spectroscopy and principal component analysis”. Lasers Med. Sci, 30: 1675-1681.
Publisher | Google Scholor - Q. Shi, S. Wang, Y. Zhou, J. Xu. (2021). “Monitoring of Fluoride Content in Drinking Water by Ion Chromatography: A Case Study in the Suzhou Urban Area, China”. Journal of AOAC International, 104(6):1533-1538.
Publisher | Google Scholor - M. Wasim, A. Tariq, R. N. Qureshi, M. A. Shafique. (2023). “Characterization of toothpastes for fluorine and other elements by INAA and ICP-OES”. Radiochimica Acta, 111(2):129-136.
Publisher | Google Scholor - G. Somer, Ş. Kalaycı, İ. Başak. (2010). “Preparation of a new solid state fluoride ion selective electrode and application”. Talanta, 80(3):1129-1132.
Publisher | Google Scholor