Research Article
Investigating the Relationship Between the Mean Heart Doses Received in Left Breast Cancer Radiation Therapy with Pro-BNP and Troponin Values and Left Ventricular Systolic Function Based on LVEF and GLS Values in Echocardiography
- Ali Akhavan 1
- Amirhosein Rayegani 1
- Azin Alizadehasl 2
- Seyed Ehsan Parhizgar 2*
- Marzieh Tajmirriahi 3
- Shadi Golchin 2
- Mahnaz Roayaei 1
- Ibrahim Abdollahpour 4
1Department of Radio oncology, School of Medicine, Cancer Prevention Research Center, Seyyed al-Shohada Hospital, Isfahan University of Medical Sciences, Isfahan, Iran.
2Cardio-Oncology Research Center, Rajaie Cardiovascular Medical and Research Center, Iran University of Medical Sciences, Tehran, Iran.
3Department of Cardiology, School of Medicine, Hypertension Center, Cardiovascular Research Institute, Nour & Ali-Asghar Hospital, Isfahan University of Medical Sciences, Isfahan, Iran.
4Assistant Professor of Epidemiology, Child Growth and Development Research Center, Research Institute for Primordial Prevention of Non-communicable Disease, Isfahan University of Medical Sciences, Isfahan, Iran.
*Corresponding Author: Seyed Ehsan Parhizgar, Cardio-Oncology Research Center, Rajaie Cardiovascular Medical and Research Center, Iran University of Medical Sciences, Tehran, Iran.
Citation: Akhavan A., Rayegani A., Alizadehasl A., Parhizgar S. E., Tajmirriahi M., et al. (2024). Investigating the Relationship Between the Mean Heart Doses Received in Left Breast Cancer Radiation Therapy with Pro-BNP and Troponin Values and Left Ventricular Systolic Function Based on LVEF and GLS Values in Echocardiography, Journal of Clinical Cardiology and Cardiology Research, BioRes Scientia Publishers. 3(2), 1-11. DOI: 10.59657/2837-4673.brs.24.030
Copyright: © 2024 Seyed Ehsan Parhizgar, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: March 27, 2024 | Accepted: May 08, 2024 | Published: May 16, 2024
Abstract
Background: The purpose of this study is to investigate the relationship between the mean heart dose received in left breast cancer radiation therapy and cardiac injury as measured by Pro-BNP and Troponin laboratory levels and left ventricular systolic function, as measured by LVEF and GLS levels in echocardiography.
Material and Methods: This research was carried out at Omid Hospital, Isfahan City, Iran, using the before and after intervention study and single-arm approach on a population of patients diagnosed with cancer of the left breast who received radiation therapy for the left breast between 2021- 2022. Troponin, Pro-BNP, GLS, and LVEF were investigated before and after intervention. The data was analyzed using the t-test and the Mann-Whitney U test. Ultimately, it was examined using version 22 of the SPSS program.
Results: This study showed a statistically significant difference in pro-BNP before and after the intervention (P<0.001). Also, we investigated the relationship between the mean heart dose, mean LAD dose, mean LV dose, radiotherapy protocol, and V25 index with the changes of Troponin, Pro-BNP, GLS, and LVEF, and we observed no statistically significant relationship between them (P>0.05).
Conclusion: Left breast radiation therapy does not affect cardiac factors like LVEF and troponin in the short term. Also, we observed Pro-BNP meaningful changes and the effect of V25 on GLS after the radiation. Studies with a larger sample size and a longer follow-up period are suggested.
Keywords: mean heart dose; breast cancer; radiation therapy; pro-BNP; troponin; LVEF; GLS; v25
Introduction
The most prevalent form of recurrent cancer and the second leading cause of death in women is breast cancer (BC). The mortality rate associated with lung cancer is the only type of cancer that is higher than that of breast cancer [1]. Radiation therapy, often known as "radiotherapy" or "RT," is the primary component of the treatment for breast cancer. As an adjuvant, radiation therapy lowers the risk of local recurrences and death [2]. The most frequent radiation therapy is applied to the full breast or chest and consists of 50 Gray (Gy) in 25 fractions, 42.5 Gy in 16 fractions, or 40 Gray in 15 fractions. This treatment is administered five days a week [3]. However, Radiotherapy to the mediastinum and chest causes increased heart damage (cardiotoxicity) through damage to the micro and macro vessels [4,5]. Before the 1990s, the magnitude and severity of radiation-induced heart disease were largely unknown.
In the 1990s, however, randomized trials in early-stage breast cancer demonstrated that radiation-related cardiac damage nearly outweighed its survival benefits. Irradiation's potential to induce cardiac disorders has received much attention as of late, which has resulted in increased attempts to lower the amount of radiation delivered to the heart [6,7]. However, the use of contemporary radiation therapy procedures, which include lowering the dose and the amount of the heart exposed to radiation in treating most cancers, has considerably reduced the incidence of this problem [8]. If a significant volume of the heart is irradiated with a high dose, it is almost certain that the irradiation will cause damage to the structures of the heart. These structures include the pericardium, the myocardium, the heart valves, the coronary vessels, the capillaries, and the conduction system. There is no question that the older radiation treatment procedures used to treat patients with thoracic cancer enhanced the morbidity and mortality associated with cardiovascular disease. During these treatments, a significant portion of the patient's heart was subjected to intense doses of radiation. As previously indicated, improvements in radiotherapy methods have significantly decreased the amount of unintentional radiation exposure to the heart. However, cardiovascular complications in breast cancer patients, which are more prevalent in left-sided than right-sided tumors, show that this danger has not been eradicated [9]. Even though pericarditis and pericardial effusion are thought to be the most common complications of radiation therapy to the heart [10], evidence gathered many years ago demonstrates that radiation-induced coronary heart disease (CHD) is the leading cause of death in patients who have undergone radiation therapy to the chest. Concerns have been raised over the potential long-term effects of radiation on the heart, particularly in individuals at an increased risk of developing ischemic disease [11].
Patients who are diagnosed with breast cancer frequently require radiation therapy to bring the disease under control. Damage to the heart muscle and cardiac structures, which appears as both functional and structural abnormalities, is one of the most serious adverse effects of radiation therapy in these patients, and it is more prevalent in patients with cancer of the left breast. Since the exposure of the heart to radiation is unavoidable in most breast cancer cases that call for radiation therapy, it is crucial to try to adjust the received radiation dose as well as evaluate the extent to which the heart has been damaged to prevent possible heart function disorders [12-14].
According to previous research findings, most studies have focused on determining the long-term and chronic cardiac side effects of radiation therapy on the left breast. Early studies, on the other hand, did not study the issues of cardiac systolic function throughout the months following radiation therapy. Therefore, by analyzing it, if these problems are diagnosed early, effective treatment may be initiated to prevent cardiac handicaps or death caused by radiation therapy. Because of this, complications such as cardiomyopathy and heart failure are significant.
Only a few studies have been done to determine the experimental factors and the clinical follow-up with echocardiography needed to accurately predict and evaluate the prognosis of cardiac problems caused by radiation therapy. In this study, an attempt was made to investigate the early findings of complications of cardiac systolic function during at least 6 months and up to 9 months after radiation therapy. This was accomplished so that effective treatment could be provided to prevent disability or cardiac deaths caused by radiation therapy from the beginning. Also, due to the significance of the side effects mentioned, it is essential to investigate the quantity of doses delivered to the heart and the changes caused by it. This will enable a comparison between the side effects' efficacy and the benefits of radiotherapy. In light of this, the purpose of this study was to investigate the relationship between the mean heart dose received in left breast cancer radiation therapy, as measured by Pro-BNP and Troponin levels, and left ventricular systolic function, as measured by LVEF and GLS levels in echocardiography.
Material and Methods
Study Population
This research was carried out at Omid Hospital in Isfahan City, Iran, using the before and after intervention study and single-arm approach on a population of patients diagnosed with cancer of the left breast who received radiation therapy for the left breast between 2021- 2022. According to the research findings by Nellessen et al., the mean and standard deviation of BNP levels before and after radiotherapy were 123 (147) and 159 (184). To calculate the difference between the two means before and after radiotherapy, they required a sample size of 10 individuals based on the method for comparing dependent means. In the study conducted by Nellessen et al., the mean and standard deviation of Troponin before and after radiotherapy were reported as 0.007 (0.008) and 0.014 (0.01), respectively [12], so a sample size of three people was required to make a comparison between Troponin levels before and after radiotherapy. According to the mean and standard deviation of LVEF before and after radiotherapy in the study of Erven et al., which were reported as 73.3 (8.3) and 70.5 (7.1), respectively [15,16], estimating the difference between LVEF before and after radiotherapy required 37 individuals, with a power of 80% and the assumption that there was a correlation of 0.6 between the before and after radiotherapy values. In our investigation, the same sample size was considered, with an estimated 20% increase in participants, meaning 45 individuals, to prevent power loss. The sample size was included in the study using convenient sampling.
The inclusion criteria for the study were to be at least 18 years old and not receiving chemotherapy (except for receiving only taxane [Docetaxel]-cyclophosphamide). In this study, patients with end-stage or metastatic malignancy, patients with two synchronous cancers, patients with a history of heart failure with LVEF≤50% or a history of valvular heart disease, advanced congenital heart disease or proven coronary artery disease, patients with a history of radiation therapy to the chest, pregnant patients with breast cancer, as well as patients receiving or with a history of receiving anthracyclines and trastuzumab drugs, were not allowed to enter/include the study. Exclusion criteria included the patient passing away at any point during the study, the patient becoming pregnant, a new case of advanced heart disease, such as heart failure, coronary or valvular heart failure, or a second cancer developing during the study that required chemotherapy or radiation therapy, and a new case of advanced heart disease.
Data collection
Echocardiographic Measurements
Transthoracic echocardiography (TTE) was performed using available tools according to the American Society of Echocardiography (ASE) recommendations [17]. All subjects were in sinus rhythm when the measurements were obtained, with analysis performed using a mean of three cardiac cycles [18]. Apical four, two-, and three-chamber views were obtained for all measurements. LV end-diastolic and end-systolic volumes and LV ejection fraction were measured using Simpson’s biplane method. Strain imaging was performed, analyzed, and reported per standardized recommendations5 on the Philips EPIC 7C (Philips Healthcare; Best, The Netherlands) echocardiography machine. The endocardial border of the left ventricle was traced manually throughout the end-systolic frames in all the 3 apical views [19]. Patients who met the trial participation criteria were enrolled whenever they were available. At the outset of radiation therapy, the patients were first given echocardiograms to test their systolic function.
The LVEF and GLS indexes were also examined, and quantitative Troponin I and Pro-BNP measurements were obtained. Following the 3D radiotherapy performed on the left breast, the levels of Troponin I (quantitatively) and Pro-BNP were obtained once again on the day the radiation therapy was finished. The cardiac LVEF and GLS values were examined at least six months later by echocardiography. Ultimately, these numbers were compared with mean heart dose, mean LV dose, mean LAD dose, radiotherapy protocol, and V25 index based on the DVH profile (dose-volume histogram). Eligible people, after taking a detailed history and examination by an assistant at Omid Hospital and proving the necessity of radiation therapy of the left breast based on the pathology and surgical report and also meeting the criteria for entering this study, after explaining the study to the patient and completing the consent form designed for referring patients, which includes the full details of the patient and underlying disease and his records, was referred to Chairman Hospital for echocardiography and the mentioned values were recorded. Then, they were referred to Seyed al-Shohada (Omid) Hospital for radiation therapy at the appointed time. On the day of radiation therapy, before receiving radiation, Troponin I and Pro-BNP blood tests were taken from the patients, and they were treated. After radiation therapy (conventional or hypo fractionated), blood samples were taken for Troponin I and Pro-BNP levels on the day of treatment completion. After 6 to 9 months, they were again subjected to a heart examination with echocardiography. Then, the new values were recorded and compared with the previous values based on the effect of the mean radiation dose on the heart.
Before radiotherapy, a CT scan for radiation therapy, also known as a CT simulation, was performed. After that, the left breast was chosen as the primary site for receiving radiation, and the nearby organs (organs at risk) were identified as structures at risk of radiation damage in CT scan sections using Linatech-Tigrt software. An assistant working at Omid Hospital performed treatment design (contouring) and planning. In this study, the entire heart (along with the pericardium) and the structures of the left anterior descending artery and the left ventricle were contoured based on the Michigan atlas [20]. The assistant then recorded the mean dose allowed to the heart and its components and V25 index, specified in the DVH profile. Patients were treated using either two conventional methods with a dose of 5000 cGy in 25 fractions or a hypo fractionated method with either Canadian protocols with a dose of 4250 cGy in 16 fractions [21] or the British protocol of 4000 cGy in 15 fractions [22] with a Primus linear accelerator with 6 megavolts of photon energy. Both categories were investigated as part of this research project. Boost was allowed in the protocols.
Ethical Considerations
This study was approved by the ethics committee of Isfahan University of Medical Sciences, Iran, with the code of ethics of IR.MUI.MED.REC.1400.397 and IRCT20210822052256N2. All patients were explained about the method and objectives of the study and the confidentiality of all information.
Data Analysis
After gathering data for this investigation, the researchers determined the absolute and relative frequency criteria and the frequency percentage for qualitative factors. For quantitative variables, the researchers determined the mean and standard deviation. The data was analyzed using the t-test and the Mann-Whitney U test. Ultimately, it was examined using version 22 of the SPSS program. This test meets the criteria for statistical significance with a p-value of less than 0.05.
Results
Forty-five patients participated in this study, and the mean and standard deviation of the age of these patients was 52.35±10.34. Out of all the patients, 45 (100%) were female. Other demographic information of the patients is reported in Table 1.
Table 1: Background information of patients.
| Variables | Frequency | Percentage |
| Underlying disease | ||
| Hypertension | 7 | 15.6 |
| Diabetes | 5 | 9.6 |
| Ischemic Heart Disease | 0 | 0 |
| Congenital diseases | 0 | 0 |
| Thyroid disease | 2 | 4.4 |
| Rheumatic disease | 0 | 0 |
| Other disease | 7 | 14.6 |
| Dyslipidemia | 3 | 6.7 |
| alcohol consumption | 0 | 0 |
| Cardiac familial history | 1 | 2.2 |
| Cancer familial history | 8 | 17.8 |
| Hormonal status | ||
| Luminal A | 31 | 68.9 |
| Luminal B | 9 | 88.9 |
| Triple Negative | 5 | 11.1 |
| Cancer grade | ||
| Grade I | 15 | 33.3 |
| Grade II | 25 | 55.6 |
| Grade III | 5 | 11.1 |
| Chemotherapy history | 19 | 42.2 |
| Type of hormone therapy | ||
| Tamoxifen | 14 | 32.6 |
| Letrozole | 29 | 67.4 |
| Type of radiotherapy | ||
| Conventional | 6 | 13.3 |
| Conventional + Boost | 6 | 13.3 |
| Hypo-Fractionated | 25 | 55.6 |
| Hypo-Fractionated + Boost | 8 | 17.8 |
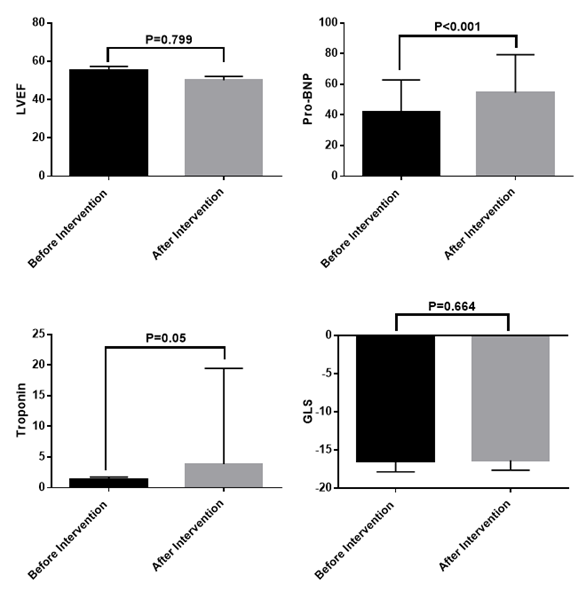
Based on the objectives of this study, the levels of pro-BNP, troponin, LVEF, and GLS of the patients before and after the intervention were investigated, the results of which are reported in Figure 1. According to the results, there was a statistically significant difference in pro-BNP before and after the intervention, so its level increased significantly after the intervention.
Figure 1: Review of information related to pro-BNP, troponin, LVEF, and GLS.
In this study, we observed the relationship between the mean heart dose, mean LAD dose, mean LV dose, radiotherapy protocol, and V25 with Troponin, Pro-BNP, GLS, and LVEF, and no significant relationship between them (P>0.05). The results of this study are reported in Table 2.
Table 2: The relationship between the average dose received by the heart and the changes in the examined parameters using linear regression.
| Variables | Outcome | B index | P-value |
| Mean heart dose received | Troponin change | 0.00032 | 0.588 |
| Pro-BNP change | 0.0098 | 0.421 | |
| GLS change | -0.001 | 0.189 | |
| LVEF change | 0.002 | 0.493 | |
| Mean LAD dose | Troponin change | 0.00016 | 0.786 |
| Pro-BNP change | 0.0069 | 0.300 | |
| GLS change | 0.0096 | 0.530 | |
| LVEF change | 0.099 | 0.297 | |
| Mean LV dose | Troponin change | -0.027 | 0.238 |
| Pro-BNP change | 0.00039 | 0.699 | |
| GLS change | 0.0094 | 0.316 | |
| LVEF change | 0.061 | 0.415 | |
| Radiotherapy protocol | Troponin change | 0.0672 | 0.439 |
| Pro-BNP change | 0.00064 | 0.819 | |
| GLS change | 0.0031 | 0.662 | |
| LVEF change | -0.00084 | 0.812 | |
| V25 index | Troponin change | 0.011 | 0.696 |
| Pro-BNP change | 0.551 | 0.370 | |
| GLS change | -0.099 | 0.093 | |
| LVEF change | 0.064 | 0.627 |
Discussion
In this study, we investigated the relationship between the mean heart dose received in left breast cancer radiation therapy with Pro-BNP, Troponin values, and left ventricular systolic function based on LVEF and GLS values in echocardiography. This study's results showed a significant difference in pro-BNP before and after the intervention, so its level increased significantly after the intervention. Also, in this study, we investigated the relationship between the mean heart dose, mean LAD dose, mean LV dose, radiotherapy protocol, and V25 index with Troponin, Pro-BNP, GLS, and LVEF changes. We observed no significant relationship between them (P>0.05), though there was a trend toward significance between V25 and GLS changes (P=0.093).
Based on the studies that have been done until now, it has been proven that radiotherapy in breast cancer patients can be associated with heart damage. In line with this, a population-based case-control study of major coronary events by Darby et al. showed that in the context of breast radiotherapy, for every 1 Gy increase in mean heart dose, a 7% increase in cardiac events occurred [23]. Also, QUANTEC recommends restricting the volume of the heart receiving at least 25 Gy (V25) to lessthan10% to keep the risk of cardiac mortality under 1% [24]. In a different population-based case-control study, the researchers found that the risk of heart failure after radiotherapy increased with a higher mean cardiac radiation dose. The participants in this study were older women who had breast cancer and had been treated with conformal radiotherapy. The most common form of heart failure was preserved ejection fraction (HFpEF), followed by a moderate reduction in HFpEF (40-49%). The likelihood of developing any form of heart failure in addition to HFpEF was increased with MCRD, and this remained the case even after risk adjustment for other factors. It is recognized that there is an elevated risk of heart disease and certain stages of cancer. In older women undergoing modern radiotherapy for breast cancer, the relative risk of HFpEF increases proportionately to the calculated MCRD, begins within a few years after radiotherapy, and is not limited to coronary heart events. When determining whether to treat breast cancer with radiation, these findings suggest that the cardiac dosage and the risk factors for heart failure should be considered. The importance of procedures that lower the cardiac dose should also be stressed [25].
On the other hand, a meta-analysis that was conducted by the Early Breast Cancer Trials Collaborative Group (EBCTCG) and published in 2005 found that cardiac-specific mortality in patients who underwent radiotherapy following breast surgery increased by 27% when compared to patients who underwent radiotherapy alone and were treated surgically. This finding was based on comparing patients who underwent radiotherapy alone and those who underwent radiotherapy following breast surgery [5]. The mean cardiac dose administered during RT for treating left breast cancer is often greater than during other radiation treatments.
Studies have shown that subclinical heart damage induced by chemotherapy or chemoradiation with muscle tension measurement, particularly with GLS based on non-invasive echocardiography, exhibits more sensitive changes in ventricular muscle tension than normal LVEF [16]. In other words, echocardiography can be used to evaluate myocardial function. GLS is a novel technology that uses automated 2D-speckle-tracking echocardiography (STE) to detect and evaluate subtle abnormalities in the systolic function of the left ventricle (LV). GLS has been proven to be a superior predictor of cardiac events compared to EF, and this approach is operator-independent and more repeatable than EF [23]. GLS is an echocardiographic method that identifies and evaluates subclinical and subtle anomalies in LV systolic function. As a result, it could be regarded as an early marker for radiation-induced heart damage. Several studies have shown that the GLS can give independent prognostic information on the morbidity and mortality of cardiovascular disease in the general population. The existence of a deteriorated LV stretch at the beginning of the study was related to an increased risk of heart failure and overall mortality over the follow-up period [26-28]. In two further trials, breast cancer survivors were evaluated for their LVEF and GLS. Researchers could not find evidence of a substantial reduction in LVEF following RT in patients with left or right breast cancer who were followed for 2 to 14 months. On the other hand, a substantial reduction in GLS was detected immediately after RT and 8 and 14 months after RT for left breast cancer survivors (but not for right breast cancer survivors), suggesting a dose-effect connection [15]. GLS has several features, some of which include its ability to predict all-cause mortality in the general population more accurately than LVEF [28], its enhanced ability to classify risk in patients who have heart failure [29], and its capacity to detect initial LV dysfunction in patients who are being treated with cardiac drugs and the following prognosis of cancer therapy-related cardiac dysfunction [30].
Traditional natural indicators, such as C-reactive protein, NT-proB-type natriuretic peptide, and troponin (TnI), can be markers of cardiac injury, particularly following radiotherapy [31-33]. In a study, the classical cardiac markers TnI, CK, CK-MBRI, and LDH were investigated as possible markers of subclinical heart diseases caused by radiation therapy. Among the many biomarkers that have emerged as an alternative method and a promising adjunct to routine echocardiography to describe the risk of cardiac dysfunction in clinically asymptomatic patients, many biomarkers have emerged as an alternative method and a promising adjunct to routine echocardiography. TnI and CK-MBRI levels were elevated one year after radiotherapy in 5 patients on the right side and 11 patients on the left side, which may indicate that these patients have had cardiac injury due to the radiotherapy [34].
Moreover, because of the low-cost method it takes, these indicators can be evaluated at closer distances, and their accuracy is not reliant on the operator's skill level. The most significant matter to take away from this is that biomarkers have demonstrated the capacity to forecast cardiac damage before it becomes clinically apparent [35-37]. In the field of cancer, for instance, serum molecules like cardiac troponins and natriuretic peptides have been investigated for their potential as biomarkers of heart injury for the past 15 years. After chemotherapy, radiation therapy, and targeted therapies, whether these biomarkers can detect individuals at risk for developing cardiac issues in adults or children has been explored. Analysis of biomarkers reveals an effective strategy, does not require the patient to undergo any unnecessary procedures, and is non-invasive for classifying individuals at risk who have had cardiotoxic therapies [32]. Besides that, despite cardiac indicators' high sensitivity, they lack much specificity [38]. The most effective cardiac indicators for acute coronary syndrome and heart failure include troponin and brain natriuretic peptides. Troponin is one of the most common cardiac markers [39]. Cardiac troponin I (TnI) and T (TnT) are extremely sensitive and precise heart injury markers. Cardiac troponins have proven to help assess acute cardiac injury in various clinical settings, including heart failure, pulmonary embolism, stroke, septic shock, and drug-induced cardiac effects. It is important to highlight that troponin have become a technique for risk categorization since the concentration of cardiac troponin is connected to the severity of myocyte damage and the following clinical consequences [40,41].
Additionally, cardiac troponins are sensitive and specific indicators that can be used to anticipate the development and degree of ventricular dysfunction [42]. Studies in which troponin has been used as a diagnostic technique for many patients treated with RT are uncommon. Some people who strive to stress the effect of radiotherapy have not been able to clinically determine the utility of troponin in predicting cardiac disease induced by radiation therapy [43,44]. However, there are a few pieces of evidence regarding the function of troponin in diagnosing cancer radiation-induced cardiotoxicity. Higher troponin levels were observed with higher doses of radiation to the heart, and this was also associated with rapid deterioration of echocardiographic muscle tension and diastolic parameters, as shown in two studies designed to investigate radiotherapy's effect on breast cancer patients [15,31].
Natriuretic peptides, such as atrial natriuretic peptides (ANP), brain natriuretic peptides (BNP), and their N-terminal prohormone constituent (NT-proBNP), have been the subject of much research and are used in the diagnosis and prognosis of acute and chronic heart failure. Chronic increases in BNP indicate increased diastolic pressure, LV wall stress, and volume overload [45,46]. Also, the concentration of NT-proBNP is linked with LVEF values and the degree of heart failure [47]. Patients undergoing chest irradiation also have elevated natriuretic peptide levels, which may indicate cardiac damage before LVEF. Individuals who underwent radiotherapy for left-sided breast cancer had levels of NT-proBNP that were considerably higher 6 months after radiation treatment than those who did not [13]. The findings of these studies are consistent with the findings of our study. The levels of BNP were shown to have significantly increased in a wide number of investigations, and NT-proBNP has both been linked to the administration of doxorubicin, epirubicin, trastuzumab, and chest radiation treatment, either individually or in conjunction with each other. Extensive research using natriuretic peptides has revealed that radiation therapy can cause heart disease even years after the treatment. Following chest radiation treatment for breast and esophageal cancer, a substantial rise in NT-proBNP was found at 9 months and 6.7 years after the treatment.
Similarly, NT-proBNP was found to connect with cardiac dosage in patients with left-sided breast cancer. D'Erico et al. discovered that there was a significant association between NT-proBNP and V3Gy (received a volume of at least 3 Gy) as well as two ratios for the heart: D15cm3/Dmean and ٪D15cm3/D50 (where Dmean is the mean dose, D50% is the value average, and D15cm3 is the minimum isodose received by 15 cm3) [48,49]. Because RT is often combined with chemotherapy, radiation to the heart has been repeatedly described as an additional risk factor for heart damage caused by anthracyclines [50]. Radiation therapy has seen significant technological advancements in recent decades. These advancements, such as conformal radiation, which reduces the amount of radiation the myocardium can absorb, are extremely helpful in lowering the risk of cardiac complications from radiation treatment [51]. According to Walker et al.'s study, the possibility of using paraclinical diagnostic factors after breast cancer radiotherapy is useful in preventing heart damage [52]. Some studies are also looking for a solution to quickly diagnose heart damage caused by breast cancer radiotherapy [53].
Limitations
The short-term follow-up period, the small sample size (the small number of patients who did not receive chemotherapy or only received CPM+taxane chemotherapy), and the two-centered nature of the study, which caused troubles to the patients and imposed costs on the researchers, are some of the limitations of this study.
Conclusion
Based on the findings of this study, it can be concluded that left breast radiation therapy lacks much effect on cardiac factors like LVEF and troponin in the short term. Also, we observed Pro-BNP meaningful changes and the role of V25 on GLS after the radiation. Studies with a larger sample size and a longer follow-up period are suggested.
Declarations
Ethics Approval and Consent to Participate
Ethics approval for the present study has been obtained from research ethics committee of Isfahan University of Medical Sciences with reference number: IR.MUI.MED.REC.1400.397
Consent for Publication
Written informed consent was obtained from all cases regarding the consent for publication of their data as a result of this study.
Availability of Data and Materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare that they have no competing interests.
Funding
This research has no source of funding to be declared.
Authors’ Contributions
Conceptualization: A. Ak, A.R; Formal analysis: S.P; Supervision: M.T; Validation: A. Al, I.A.; Visualization: S.G
Writing–original draft: A.R., S.P., M.T., I.A.
References
- Group UCSW. (2014). United States cancer statistics: 1999-2011 incidence and mortality web-based report. Atlanta GA Dep Health Hum Serv Cent Dis Control Prev Natl Cancer Inst.
Publisher | Google Scholor - Abe O, Abe R, Enomoto K, Kikuchi K, Koyama H, et al. (2005). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomized trials. Lancet. 366(9503):2087-2106.
Publisher | Google Scholor - Koulis TA, Phan T, Olivotto IA. (2015). Hypofractionated whole breast radiotherapy: current perspectives. Breast Cancer Targets Ther. 7:363.
Publisher | Google Scholor - Senkus-Konefka E, Jassem J. (2007). Cardiovascular effects of breast cancer radiotherapy. Cancer Treat Rev. 33(6):578-593.
Publisher | Google Scholor - Cuzick J, Stewart H, Rutqvist L, Houghton J, Edwards R, et al. (1994). Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol. 12(3):447-453.
Publisher | Google Scholor - Sardaro A, Petruzzelli MF, D’Errico MP, Grimaldi L, Pili G, et al. (2012). Radiation-induced cardiac damage in early left breast cancer patients: risk factors, biological mechanisms, radiobiology, and dosimetric constraints. Radiother Oncol. 103(2):133-142.
Publisher | Google Scholor - Group EBCTC. Radiotherapy for early breast cancer. 2002.
Publisher | Google Scholor - Patel CG, Michaelson E, Chen YH, Silver B, Marcus KJ, et al. (2018). Reduced mortality risk in the recent era in early-stage Hodgkin lymphoma patients treated with radiation therapy with or without chemotherapy. Int J Radiat Oncol Biol Phys. 100(2):498-506.
Publisher | Google Scholor - Marks L, Constine LS, Adams J, Schild SE. (2017). Cardiotoxicity of radiation therapy for breast cancer and other malignancies. UpToDate.
Publisher | Google Scholor - Boice Jr JD. (2007). An affair of the heart. Oxford University Press.
Publisher | Google Scholor - Loyer EM, Delpassand ES. (1993). Radiation-induced heart disease: imaging features. In: Seminars in roentgenology. Elsevier; p. 321-332.
Publisher | Google Scholor - Gagliardi G, Lax I, Rutqvist LE. (2001). Partial irradiation of the heart. In: Seminars in radiation oncology. Elsevier; p. 224-233.
Publisher | Google Scholor - Nellessen U, Zingel M, Hecker H, Bahnsen J, Borschke D. (2010). Effects of radiation therapy on myocardial cell integrity and pump function: which role for cardiac biomarkers? Chemotherapy. 56(2):147-152.
Publisher | Google Scholor - Adams MJ, Hardenbergh PH, Constine LS, Lipshultz SE. (2003). Radiation-associated cardiovascular disease. Crit Rev Oncol Hematol. 45(1):55-75.
Publisher | Google Scholor - Erven K, Florian A, Slagmolen P, Sweldens C, Jurcut R, et al. (2013). Subclinical Cardiotoxicity Detected by Strain Rate Imaging up to 14 months After Breast Radiation Therapy. Int J Radiat Oncol Biol Phys. 85(5):1172-1178.
Publisher | Google Scholor - Erven K, Jurcut R, Weltens C, Giusca S, Ector J, et al. (2011). Acute radiation effects on cardiac function detected by strain rate imaging in breast cancer patients. Int J Radiat Oncol Biol Phys. 79(5):1444-1451.
Publisher | Google Scholor - Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, et al. (2015). Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 28(1):1-39.e14.
Publisher | Google Scholor - Tajmirriahi M, Salari P, Saadatnia M. (2022). Left atrial function index in embolic stroke of undetermined source: A case-control study. Neurol Asia. 27(2):275-279.
Publisher | Google Scholor - Sharma YP, Batta A, Kaur N, et al. (2022). Accuracy of Global Longitudinal and Territorial Longitudinal Strain in Determining Myocardial Viability in Comparison to Single-Photon Emission Computed Tomography in Out of Window Period Anterior Wall Myocardial Infarction Patients. Anatol J Cardiol. 26(8):637-644.
Publisher | Google Scholor - Feng M, Moran JM, Koelling T, Chughtai A, Chan JL, et al. (2011). Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 79(1):10-18.
Publisher | Google Scholor - Whelan T, MacKenzie R, Julian J, Levine M, Shelley W, et al. (2002). Randomized Trial of Breast Irradiation Schedules After Lumpectomy for Women with Lymph Node-Negative Breast Cancer. JNCI J Natl Cancer Inst. 94(15):1143-1150.
Publisher | Google Scholor - Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, et al. (2009). The UK Standardization of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet. 371(9618):1098-1107.
Publisher | Google Scholor - Jacob S, Pathak A, Franck D, Latorzeff I, Jimenez G, et al. (2016). Early detection and prediction of cardiotoxicity after radiation therapy for breast cancer: the BACCARAT prospective cohort study. Radiat Oncol. 11(1):54.
Publisher | Google Scholor - Moiseenko V, Einck J, Murphy J, Ödén J, Bjöhle J, et al. (2016). Clinical evaluation of QUANTEC guidelines to predict the risk of cardiac mortality in breast cancer patients. Acta Oncol. 55(12):1506-1510.
Publisher | Google Scholor - Saiki H, Petersen IA, Scott CG, Bailey KR, Dunlay SM, et al. (2017). Risk of heart failure with preserved ejection fraction in older women after contemporary radiotherapy for breast cancer. Circulation. 135(15):1388-1396.
Publisher | Google Scholor - Cheng Susan, McCabe Elizabeth L., Larson Martin G., Merz Allison A., Osypiuk Ewa, et al. Distinct Aspects of Left Ventricular Mechanical Function Are Differentially Associated with Cardiovascular Outcomes and All‐Cause Mortality in the Community. J Am Heart Assoc. 4(10):e002071.
Publisher | Google Scholor - Biering-Sørensen Tor, Biering-Sørensen Sofie Reumert, Olsen Flemming Javier, Sengeløv Morten, Jørgensen Peter Godsk, et al. (2017). Global Longitudinal Strain by Echocardiography Predicts Long-Term Risk of Cardiovascular Morbidity and Mortality in a Low-Risk General Population. Circ Cardiovasc Imaging. 10(3):e005521.
Publisher | Google Scholor - Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community‐based cohort - Russo - 2014 - European Journal of Heart Failure - Wiley Online Library, 2020.
Publisher | Google Scholor - Cho GY, Marwick TH, Kim HS, Kim MK, Hong KS, et al. (2009). Global 2-Dimensional Strain as a New Prognosticator in Patients with Heart Failure. J Am Coll Cardiol. 54(7):618-624.
Publisher | Google Scholor - Sawaya Heloisa, Sebag Igal A., Plana Juan Carlos, Januzzi James L., Ky Bonnie, et al. (2012). Assessment of Echocardiography and Biomarkers for the Extended Prediction of Cardiotoxicity in Patients Treated with Anthracyclines, Taxanes, and Trastuzumab. Circ Cardiovasc Imaging. 5(5):596-603.
Publisher | Google Scholor - Skyttä T, Tuohinen S, Boman E, Virtanen V, Raatikainen P, et al. (2015). Troponin T-release associates with cardiac radiation doses during adjuvant left-sided breast cancer radiotherapy. Radiat Oncol. 10(1):141.
Publisher | Google Scholor - Tian S, Hirshfield KM, Jabbour SK, Toppmeyer D, Haffty BG, et al. (2014). Serum biomarkers for the detection of cardiac toxicity after chemotherapy and radiation therapy in breast cancer patients. Front Oncol. 4:277.
Publisher | Google Scholor - D’Errico MP, Grimaldi L, Petruzzelli MF, Gianicolo EAL, Tramacere F, et al. (2012). N-Terminal Pro-B–Type Natriuretic Peptide Plasma Levels as a Potential Biomarker for Cardiac Damage After Radiotherapy in Patients with Left-Sided Breast Cancer. Int J Radiat Oncol Biol Phys. 82(2):e239-246.
Publisher | Google Scholor - Zaher E, Fahmy E, Mahmoud K, El Kerm Y, Auf M. (2018). Assessment of the onset of radiation-induced cardiac damage after radiotherapy of breast cancer patients. Alex J Med. 54(4):655-660.
Publisher | Google Scholor - Cardinale D, Salvatici M, Sandri MT. (2011). Role of biomarkers in cardio-oncology. Clin Chem Lab Med CCLM. 49(12):1937-1948.
Publisher | Google Scholor - Dolci A, Dominici R, Cardinale D, Sandri MT, Panteghini M. (2008). Biochemical markers for prediction of chemotherapy-induced cardiotoxicity: systematic review of the literature and recommendations for use. Am J Clin Pathol. 130(5):688-695.
Publisher | Google Scholor - Cardinale D, Sandri MT. (2010). Role of biomarkers in chemotherapy-induced cardiotoxicity. Prog Cardiovasc Dis. 53(2):121-129.
Publisher | Google Scholor - Christenson ES, James T, Agrawal V, Park BH. (2015). Use of biomarkers for the assessment of chemotherapy-induced cardiac toxicity. Clin Biochem. 48(4-5):223-235.
Publisher | Google Scholor - Tan LL, Lyon AR. (2018). Role of biomarkers in prediction of cardiotoxicity during cancer treatment. Curr Treat Options Cardiovasc Med. 20(7):55.
Publisher | Google Scholor - Panteghini M. (2004). Role and importance of biochemical markers in clinical cardiology. Eur Heart J. 25(14):1187-1196.
Publisher | Google Scholor - Adamcova M, Šterba M, Šimunek T, Potacova A, Popelova O, et al. (2005). Troponin as a marker of myocardiac damage in drug-induced cardiotoxicity. Expert Opin Drug Saf. 4(3):457-472.
Publisher | Google Scholor - Cardinale D, Sandri MT, Colombo A, Colombo N, Boeri M, et al. (2004). Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 109(22):2749-2754.
Publisher | Google Scholor - Kozak KR, Hong TS, Sluss PM, Lewandrowski EL, Aleryani SL, et al. (2008). Cardiac blood biomarkers in patients receiving thoracic (chemo) radiation. Lung Cancer. 62(3):351-355.
Publisher | Google Scholor - Hughes-Davies L, Sacks D, Rescigno J, Howard S, Harris J. (1995). Serum cardiac troponin T levels during treatment of early-stage breast cancer. J Clin Oncol. 13(10):2582-2584.
Publisher | Google Scholor - Masson S, Latini R. (2008). Amino-terminal pro–B-type natriuretic peptides and prognosis in chronic heart failure. Am J Cardiol. 101(3):S56-60.
Publisher | Google Scholor - Grewal J, McKelvie RS, Persson H, Tait P, Carlsson J, et al. (2008). Usefulness of N-terminal pro–brain natriuretic peptide and brain natriuretic peptide to predict cardiovascular outcomes in patients with heart failure and preserved left ventricular ejection fraction. Am J Cardiol. 102(6):733-737.
Publisher | Google Scholor - Roberts NJ, Norris AL, Petersen GM, Bondy ML, Brand R, et al. (2016). Whole genome sequencing defines the genetic heterogeneity of familial pancreatic cancer. Cancer Discov. 6(2):166-175.
Publisher | Google Scholor - Jingu K, Nemoto K, Kaneta T, Oikawa M, Ogawa Y, et al. (2007). Temporal change in brain natriuretic Peptide after radiotherapy for thoracic esophageal cancer. Int J Radiat Oncol Biol Phys. 69(5):1417-1423.
Publisher | Google Scholor - Perik PJ, De Vries EG, Boomsma F, van der Graaf WT, Sleijfer DT, et al. (2005). Use of natriuretic peptides for detecting cardiac dysfunction in long-term disease-free breast cancer survivors. Anticancer Res. 25(5):3651-3657.
Publisher | Google Scholor - Shapiro CL, Hardenbergh PH, Gelman R, Blanks D, Hauptman P, et al. (1998). Cardiac effects of adjuvant doxorubicin and radiation therapy in breast cancer patients. J Clin Oncol. 16(11):3493-3501.
Publisher | Google Scholor - Patt DA, Goodwin JS, Kuo YF, Freeman JL, Zhang DD, et al. (2005). Cardiac morbidity of adjuvant radiotherapy for breast cancer. J Clin Oncol. 23(30):7475-7482.
Publisher | Google Scholor - Walker V, Crijns A, Langendijk J, Spoor D, Vliegenthart R, et al. (2018). Early Detection of Cardiovascular Changes After Radiotherapy for Breast Cancer: Protocol for a European Multicenter Prospective Cohort Study (Medirad Early Heart Study). JMIR Res Protoc. 7(10):e178.
Publisher | Google Scholor - Zhu D, Li T, Zhuang H, Cui M. (2022). Early Detection of Cardiac Damage by Two-Dimensional Speckle Tracking Echocardiography After Thoracic Radiation Therapy: Study Protocol for a Prospective Cohort Study. Front Cardiovasc Med. 8:735265.
Publisher | Google Scholor