Review Article
Invasive Fungal Disease Associated with Covid-19 Infection
- Negeri Debela *
- Solome Nekahiwot
Department of Medical Laboratory Science, College of Health Sciences, Arsi University, Asella, Ethiopia.
*Corresponding Author: Negeri Debela, Department of Medical Laboratory Science, College of Health Sciences, Arsi University, Asella, Ethiopia.
Citation: Debela N., Nekahiwot S. (2024). Invasive Fungal Disease Associated with Covid-19 Infection, International Journal of Clinical and Surgical Pathology, BioRes Scientia Publishers. 1(1):1-8. DOI: 10.59657/ijcsp.brs.24.002
Copyright: © 2024 Negeri Debela, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: April 25, 2024 | Accepted: July 03, 2024 | Published: July 10, 2024
Abstract
COVID-19, caused by the SARS-CoV-2 virus, primarily affects the lungs but impacts multiple organ systems. Patients with severe COVID-19, especially those hospitalized in intensive care units, are at high risk of developing secondary infections from fungi, bacteria, and other viruses due to a combination of factors. These include a disrupted immune response, prolonged hospital stays, and the use of immunosuppressive drugs. Several types of fungal infections, including aspergillosis, candidiasis, mucormycosis, Cryptococcus, pneumocystis, and endemic fungi, have been reported in COVID-19 patients. Patients with severe COVID-19 are particularly prone to developing aspergillosis due to impaired immunity and underlying lung disease. Those with prolonged hospitalization, the use of broad-spectrum antibiotics, and impaired immunity are at high risk of Candida. Those with diabetes who are taking corticosteroids are also at high risk of mucormycosis. Diagnosing invasive fungal diseases in COVID-19 patients is challenging due to overlapping symptoms but is critical for treatment. A combination of microbiological methods, molecular techniques and histopathology is often needed for an accurate diagnosis. Enhanced monitoring, optimized treatment guidelines, and antifungal therapies will be essential as we continue to address this health crisis. Invasive fungal infections highlight the need for a coordinated 'One Health' approach to emerging infectious diseases.
Keywords: infectious diseases; mucormycosis; COVID-19; pathophysiology
Introduction
COVID-19, caused by the novel coronavirus SARS-CoV-2, first emerged in Wuhan, China, in late 2019 and has since spread globally, leading to a pandemic of unprecedented scale in recent history [1]. The virus primarily affects the respiratory system, but its impact extends to multiple organ systems, leading to a wide range of clinical manifestations. The severity of COVID-19 varies, with some individuals remaining asymptomatic, while others develop severe disease requiring hospitalization and intensive care [2]. A significant concern in managing severe COVID-19 cases is the occurrence of coinfections and superinfections. Coinfections refer to the simultaneous infection of a host by multiple pathogenic species, while superinfections refer to secondary infections that occur during or after treatment of a primary infection [3]. These additional infections can complicate the clinical course, prolong hospital stays, and increase mortality rates.
Coinfections with other respiratory viruses, such as influenza, have been reported in COVID-19 patients. These coinfections can exacerbate respiratory symptoms and potentially lead to more severe disease outcomes [4]. Bacterial coinfections, particularly with pathogens such as Streptococcus pneumoniae and Haemophilus influenzae, can also occur and contribute to disease severity [5]. Superinfections often occur in hospital settings, particularly in patients who are critically ill and require mechanical ventilation [6]. Among the various pathogens contributing to coinfections and superinfections in COVID-19 patients, fungi have emerged as significant players. Fungal infections, particularly invasive infections, are a serious concern in immunocompromised individuals and those with severe illnesses, such as COVID-19 [7]. These infections can be caused by a variety of fungal species, including Aspergillus, Candida, and Mucorales, and can lead to conditions such as invasive aspergillosis, candidiasis, and mucormycosis, respectively [8]. In the following sections, we will delve more deeply into the relationship between COVID-19 and fungal infections, exploring the pathophysiology, specific fungal pathogens, and implications for patient management and outcomes.
COVID-19 and Fungal Diseases
Fungal diseases are caused by fungi, a diverse group of microorganisms that can cause a wide range of infections, from superficial skin conditions to life-threatening systemic diseases. The most common types of fungal infections include candidiasis, aspergillosis, and cryptococcosis. The prevalence of fungal diseases varies widely depending on the specific type of infection and the population being studied. Risk factors for fungal diseases include immunosuppression, prolonged hospitalization, and the use of certain medications, such as corticosteroids and antibiotics [9]. COVID-19 has been associated with increased susceptibility to fungal infections. This is likely due to a combination of factors, including immune system dysregulation caused by the virus, prolonged hospitalization, and the use of immunosuppressive drugs in the treatment of severe COVID-19 [6]. A systematic review and meta-analysis by Musuuza et al. revealed that as many as 19% of patients with COVID-19 had coinfections, and 24% had superinfections. Of these fungal coinfections, 4% were coinfections, and 8% were superinfections. The presence of either coinfection or superinfection was associated with poor outcomes, including increased mortality [10].
Pathophysiology/Mechanisms of Fungal Infections in COVID-19 Patients
The pathophysiology of fungal infections in COVID-19 patients is complex and multifactorial. Several mechanisms have been proposed to explain the increased susceptibility to fungal infections in these patients. The inclusion criteria were as follows:
Immune System Dysregulation
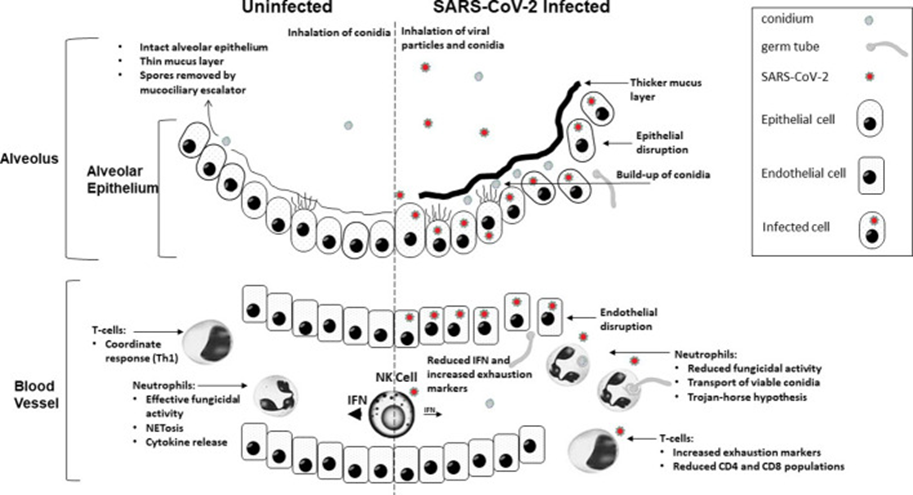
COVID-19 has the potential to disrupt the immune system, making it difficult for the body to effectively combat fungal pathogens. This disruption is characterized by a hyperinflammatory state, often referred to as a "cytokine storm," and lymphopenia, which is a reduction in T cells (Figure 1). Cytokine storms can lead to tissue damage, creating an environment conducive to fungal invasion. Moreover, lymphopenia can weaken cell-mediated immunity, a crucial component in controlling fungal infections [11].
Figure 1: The mechanisms of COVID-19 infection contribute to increased susceptibility to invasive fungal disease.
Innate antifungal immunity can be compromised in three phases due to COVID-19. Initially, severe damage to the respiratory epithelium caused by an excessive inflammatory response to the virus, coupled with limited mucociliary clearance, provides an opportunity for fungal tissue invasion. This situation is exacerbated by impaired phagocytosis of conidia, which is due to the downregulation of genes associated with phagocytic opsonization, recognition, and conidial killing. Finally, once the conidia have bypassed these defenses, the control of hyphal growth is compromised due to a decrease in neutrophil fractions [12].
Prolonged Hospitalization
Prolonged hospitalization, particularly in intensive care units (ICUs), can increase the risk of fungal infections. ICUs are high-risk environments for fungal infections due to factors such as the use of invasive devices (e.g., ventilators, central venous catheters), broad-spectrum antibiotics, and parenteral nutrition. These factors can disrupt the normal microbiota and create a favorable environment for fungal colonization and infection (7). Moreover, the immunomodulatory effects of antibiotics, such as azithromycin, can inhibit neutrophils and the innate immune response [13].
Use of Immunosuppressive Drugs
The use of immunosuppressive drugs, such as corticosteroids and tocilizumab, in the treatment of severe COVID-19 can also increase the risk of fungal infections. Corticosteroids can suppress the immune system and impair the body's ability to fight fungal pathogens. Dexamethasone, a corticosteroid, has been widely used to treat severe COVID-19 due to its ability to reduce inflammation and lung damage. However, its use has been associated with an increased risk of secondary infections, including fungal infections [14].
Common Fungal Infections in COVID-19 Patients
Several types of fungal infections have been reported in COVID-19 patients, including aspergillosis, candidiasis, mucormycosis, endemic mycoses, pneumocytosis and cryptococcosis [8].
Aspergillus
The genus Aspergillus can cause a range of illnesses in humans, from allergic reactions to life-threatening invasive infections. A. fumigatus is the most common species causing disease in humans. Patients with severe COVID-19, especially those requiring intensive care, are at high risk of developing secondary Aspergillus infections, including invasive pulmonary aspergillosis (IPA), due to their immunocompromised state and underlying lung disease [8].
Aspergillus is ubiquitous in the environment and is found in soil, water, and decaying vegetation. Most people inhale Aspergillus spores daily without harm, but immunocompromised individuals are susceptible to infection [15]. The syndromes of pulmonary aspergillosis complicating severe viral infections are distinct from classic IPA. In particular, IPA, which is associated with hematologic malignancies and transplantation, is most frequently encountered in patients with neutropenia and other immune-compromised individuals. Numerous studies have shown that influenza-associated pulmonary aspergillosis (IAPA) is associated with respiratory epithelium damage [16]. COVID-19 is linked to widespread lung parenchymal illness, including diffuse alveolar damage, hyaline membrane development, interstitial lymphocyte infiltration, and the production of vascular microthrombi. These lung abnormalities can take weeks to heal, providing an appropriate environment for fungal growth [16]. The first cases of COVID-19-associated pulmonary aspergillosis (CAPA) were reported in China in early 2020. Since then, multiple case series and cohort studies have highlighted the importance of this potentially life-threatening secondary infection, which is sometimes caused by azole-resistant Aspergillus spp. [17].
Several studies have shown that steroid and other immune-modulatory therapies are linked to an increased risk of a similar syndrome associated with severe COVID-19 [16]. CAPA is often limited to invasive airway growth for several days before it becomes angioinvasive, and patients may present with nonneutropenic disease. However, once CAPA becomes angioinvasive and produces a positive serum GM, mortality is more than 80%, even if systemic antifungal therapy is provided [17]. Van Arkel et al. reported Aspergillus species in 18% of samples from patients in the Netherlands, with 12% meeting the criteria for probable IPA [18]. Similarly, Alanio et al. reported Aspergillus species in 22% of COVID-19 patients in France, with 8% having proven or probable IPA [19]. Risk factors for developing IPA in COVID-19 patients include the use of corticosteroids, severe lymphopenia, chronic lung disease, older age and mechanical ventilation [20].
Candida
Candida is a genus of yeasts that can cause infections in humans, ranging from superficial to life-threatening invasive disease. Candida albicans is the most common species causing infection, although other species, such as C. glabrata and C. auris, are emerging as pathogens [21]. Patients with severe COVID-19, especially those requiring intensive care, are at high risk of developing secondary Candida infections due to prolonged hospitalization, the use of broad-spectrum antibiotics, and impaired immunity [8]. Candida is part of the normal human microbiota, colonizing the gastrointestinal and genitourinary tracts, although overgrowth can lead to disease. Preventing candidiasis infections has long been a medical challenge, and COVID-19-associated candidiasis (CAC) is currently posing new challenges. The most common types of Candida infections in COVID-19 patients are invasive candidiasis and candidemia [22].
Invasive Candida infections in patients with COVID-19 in the ICU were first described shortly after the emergence of SARS-CoV-2; invasive Candida infections impose considerable comorbidity. Of particular concern are outbreaks of severe Candida auris infections in patients with COVID-19 in the ICU. C. auris is difficult to distinguish from other Candida species and is often resistant to many or all antifungal drugs. It can persist on surfaces; unlike other invasive fungal infections (IFIs), it can be transmitted in healthcare facilities [17]. In India, Candida species were the third most common pathogens (4.1%) of secondary bloodstream infections among patients with COVID-19, followed by Klebsiella pneumoniae (10.9%) and Acinetobacter baumannii (8.8%) [23]. Zhou et al. conducted a study in Wuhan, China, and detected Candida spp. in 5% of respiratory specimens from ICU patients with COVID-19 [24]. In a similar study in Italy, Giacobbe et al. reported Candida spp. in 9% of secondary infections in COVID-19 patients [25]. Risk factors for infections with Candida spp. include central venous catheters, total parenteral nutrition, renal replacement therapy, diabetes, and corticosteroid use [7,26]. Prior exposure to antifungals is a major risk factor for C. auris bloodstream infection. Tocilizumab use has also been linked to C. auris fungemia in patients with COVID-19 [17].
Mucorales
Mucormycosis is an invasive fungal infection caused by fungi in the order Mucorales, including Rhizopus, Mucor, and Rhizomucor species. These fungi are ubiquitous in the environment, and infection occurs by the inhalation of spores. Patients with severe COVID-19, especially those with diabetes who are receiving corticosteroids, are at high risk of developing mucormycosis due to impaired immunity and hyperglycemia [27]. Mucormycosis, colloquially called the deadly ‘black fungus', is globally the most common infection, and the greatest number of cases were reported in India, with dramatic increases compared with pre-COVID-19 rates in which the Central Government of India declared a mucormycosis epidemic on 10 May 2021 [17]. The outbreak is suspected to be due to India’s large diabetic population, environmental factors, including the tropical and subtropical humid climates with the presence of Mucorales spores, and practice variations in the use of corticosteroids [28]. Estimates of incidence rates in other countries are scarce, although COVID-19-associated Mucorales (CAM) cases have been described in countries in Asia, Europe and South, Middle and North America [17].
In a multicenter epidemiologic study, Patel et al. compared mucormycosis cases from January to May in 2019, 2020, and during India's COVID-19 peak in 2021. They observed a 2.1-fold increase in cases in 2021, suggesting an association between the COVID-19 surge and the increased risk of mucormycosis [29]. Moorthy et al. reported that 8% of COVID-19 patients receiving oxygen therapy and 20% of those on mechanical ventilation in India developed mucormycosis [30]. Similarly, Ardehali et al. identified 12 cases of mucormycosis among 235 COVID-19 patients in Iran, with most of these patients having diabetes and receiving corticosteroids [31]. Risk factors for mucormycosis in COVID-19 patients include diabetes mellitus, hematological malignancies, organ transplants, corticosteroid use, severe lymphopenia, and mechanical ventilation [27]. In addition, COVID-19 can cause glucocorticoid-induced diabetes, and entry of the virus into pancreatic cells, which harbor angiotensin converting enzyme 2 (ACE 2), may damage beta cells, leading to insulin deficiency management and fueling the flames [28,32]. The ability of the SARS-CoV-2 spike glycoprotein to mimic 'hepcidin' can lead to hyperserotonemia in patients with severe COVID-19, causing an accumulation of intracellular iron, tissue injury, and the release of free iron into the blood, potentially stimulating Mucorales fungal pathophysiology [28].
The most common manifestation of mucormycosis in COVID-19 patients is rhino-orbital mucormycosis (ROM) or rhino-orbital cerebral mucormycosis (ROCM) infection, characterized by facial swelling, proptosis, and ophthalmoplegia. Pulmonary mucormycosis manifests as cough, chest pain, hemoptysis, and pleural effusion. Gastrointestinal mucormycosis presents with abdominal pain, gastrointestinal bleeding, and perforation. All of the abovementioned infections might evolve into the disseminated type of mucormycosis, which involves two or more widely distant organ systems [28]. The mortality rates differ depending on the clinical manifestation. COVID-19-associated ROM has a mortality rate of 14%, whereas pulmonary or disseminated mucormycosis has a mortality rate greater than 80% [17].
Other invasive fungal infections associated with COVID-19
Cryptococcosis is historically observed in patients with AIDS, malignancies, transplants, and other sources of immunosuppression. To date, several cases of COVID-19-associated cryptococcosis have been reported [8]. COVID-19-associated Cryptococcus infections typically present as meningoencephalitis and pneumonia. Patients often have underlying conditions such as HIV or use of corticosteroids [33]. Chastin D, et al., Patients with COVID-19 and cryptococcosis were more likely to be male, have pulmonary involvement, and require ICU admission. COVID-19 patients also have increased rates of respiratory failure, acute kidney injury, and mortality [34]. Several cases of COVID-19-associated Pneumocystis jirovecii have been reported. The presentation of severe COVID-19 can be clinically indistinguishable from Pneumocystis pneumonia (PCP), as patients present with bilateral ground-glass opacities and lymphopenia [8]. Gentile I, et al., identified five cases of PJP in non-HIV patients who recovered from COVID-19. They diagnosed PJP based on clinical symptoms, radiological findings, and the detection of P. Jirovecii DNA in respiratory samples. All patients had severe COVID-19 and were treated with corticosteroids. The researchers concluded that PJP should be considered in the differential diagnosis of patients with severe COVID-19 and worsening respiratory symptoms during the recovery phase, especially if they receive prolonged corticosteroid therapy [35].
There is emerging evidence that COVID-19 patients may be at greater risk of coinfection or reactivation of dimorphic fungi. Borges et al. investigated the association between COVID-19 and dimorphic fungal coinfections in hospitalized patients in Brazil. This study reviewed the medical records of 673 patients hospitalized with COVID-19. Of these, 38 patients (5.7%) had positive fungal cultures, with the most common fungi being Paracoccidioides spp. (57.9%) and Histoplasma capsulatum (39.5%). The immunosuppression caused by COVID-19, the use of immunomodulatory drugs, and lung damage may increase susceptibility to dimorphic fungi in these patients [36].
Laboratory Diagnosis
The diagnosis of IFDs in COVID-19 patients can be challenging due to overlapping clinical features with COVID-19 pneumonia, but early diagnosis and treatment are critical for reducing morbidity and mortality [37-39]. Microbiological methods such as direct microscopy, culture, and antigen testing are commonly used for the diagnosis of major IFDs such as aspergillosis, candidiasis, and mucormycosis. Direct microscopic examination of respiratory samples, blood, and tissues using stains such as potassium hydroxide (KOH) and calcofluor white can detect fungal elements and provide a quick preliminary diagnosis. However, the sensitivity is low [40]. Culture using selective fungal media such as Sabouraud dextrose agar remains the gold standard but can take 2-3 weeks for slow-growing fungi such as Aspergillus. Antigen tests such as galactomannan (for Aspergillus) and 1,3-beta-D-glucan (for Candida and Aspergillus) have good sensitivity and specificity and provide results within a day. Molecular methods such as polymerase chain reaction (PCR) can also aid in diagnosis, especially for mucormycosis [41].
Pathological examination using stains such as Gomori Methenamine Silver (GMS), periodic acid–Schiff (PAS), and Fontana-Masson can detect fungal hyphae in tissue specimens. Histopathology provides a definitive diagnosis but requires invasive procedures to obtain specimens. Nonculture-based methods such as next-generation sequencing of respiratory samples show promise for the diagnosis of a range of fungi but require further validation [41]. Due to their low sensitivity and specificity, serological tests for identifying fungal antibodies are not helpful for diagnosis, but they may be useful in epidemiological studies. ELISA, western blot, and other methods are used to detect fungal antibodies; these methods are retrospective diagnostic methods that are unable to distinguish between present and past infections and are used for the diagnosis of aspergillosis, candidiasis, and cryptococcosis [40,41]. Radiological features such as halo signs or reverse halo signs may suggest invasive aspergillosis but lack specificity. The use of bronchoalveolar lavage (BAL) to obtain respiratory samples for microbiological and pathological tests remains the most useful diagnostic procedure for detecting IFDs in COVID-19 patients [41].
Management and Treatment of Fungal Infection
The management of invasive fungal infections in COVID-19 patients requires aggressive diagnostic and treatment approaches given the high mortality associated with these secondary infections. For aspergillosis, voriconazole is the primary treatment, with liposomal amphotericin B as an alternative. Isavuconazole or itraconazole can also be used. For candidiasis, echinocandins (caspofungin, micafungin, and anidulafungin) are preferred, along with fluconazole for less ill patients or as a step-down therapy. However, only echinocandins treat multidrug-resistant Candida auris, which requires strict isolation and screening [23]. Mucormycosis requires early surgical debridement and antifungals. For neutropenic or high-risk patients, posaconazole prophylaxis may help. Amphotericin B, liposomal amphotericin B, and posaconazole were used to treat mucormycosis, and isavuconazole was used for salvage [17]. Pneumocystis jirovecii pneumonia can be treated with trimethoprim-sulfamethoxazole and steroids [8].
Conclusion
Invasive fungal infections pose a serious threat to patients with severe COVID-19 and contribute significantly to morbidity and mortality. COVID-19 patients, especially those requiring intensive care, are highly susceptible to opportunistic fungal pathogens due to immune dysregulation, prolonged hospitalization, and the use of immunosuppressive drugs. Aspergillus, Mucorales, Candida, Cryptococcus, Pneumocystis jirovecii and dimorphic fungi have been reported to cause secondary infections in COVID-19 patients, manifesting as pneumonia, sinusitis, meningitis and disseminated disease. Early diagnosis and prompt antifungal therapy are critical for improving outcomes in COVID-19 patients with invasive fungal infections. A high index of suspicion, microbiological workup of respiratory and other specimens, and empirical antifungal treatment may be warranted in some cases. Clinicians should be aware of the local epidemiology of fungal pathogens to determine appropriate antifungal regimens. Enhanced surveillance, optimized treatment guidelines, and novel antifungal therapies will be crucial as we continue to battle this global health crisis. Overall, invasive fungal infections associated with COVID-19 highlight the need for a 'One Health' perspective in tackling emerging infectious diseases.
List of Abbreviations
CAC- COVID-19-associated candidiasis
CAPA- COVID-19-associated pulmonary aspergillosis
ICUs- Intensive care units
IFIs- Invasive fungal infections
IPA- Invasive pulmonary aspergillosis
PCP- Pneumocystis pneumonia
ROCM- Rhino-orbital cerebral mucormycosis
ROM- Rhino-orbital mucormycosis
Conflict of Interest
The authors declare no conflicts of interest.
References
- Zhu N, Zhang D, Wang W, Li X, Yang B, et al. (2020). A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 382(8):727-733.
Publisher | Google Scholor - Huang C, Wang Y, Li X, Ren L, Zhao J, et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 395(10223):497-506.
Publisher | Google Scholor - Rawson TM, Moore LSP, Zhu N, Ranganathan N, Skolimowska K, et al. (2020). Bacterial and Fungal Coinfection in Individuals with Coronavirus: A Rapid Review to Support COVID-19 Antimicrobial Prescribing. Clin Infect Dis. 71(9):2459-2468.
Publisher | Google Scholor - Kim D, Quinn J, Pinsky B, Shah NH, Brown I. (2020). Rates of Coinfection Between SARS-CoV-2 and Other Respiratory Pathogens. JAMA. 323(20):2085-2086.
Publisher | Google Scholor - Garcia-Vidal C, Sanjuan G, Moreno-García E, Puerta-Alcalde P, Garcia-Pouton N, et al. (2021). Incidence of coinfections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 27(1):83-88.
Publisher | Google Scholor - Arastehfar A, Carvalho A, van de Veerdonk FL, Jenks JD, Koehler P, et al. (2020). COVID-19 Associated Pulmonary Aspergillosis (CAPA)-From Immunology to Treatment. J Fungi. 6(2).
Publisher | Google Scholor - Koehler P, Bassetti M, Chakrabarti A, Chen SCA, Colombo AL, et al. (2021). Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 21(6):149-162.
Publisher | Google Scholor - Shishido AA, Mathew M, Baddley JW. (2022). Overview of COVID-19-Associated Invasive Fungal Infection. Curr Fungal Infect Rep. 16(3):87-97.
Publisher | Google Scholor - Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, et al. (2012). Hidden killers: human fungal infections. Sci Transl Med. 4(165):165-173.
Publisher | Google Scholor - Musuuza JS, Watson L, Parmasad V, Putman-Buehler N, Christensen L, et al. (2021). Prevalence and outcomes of coinfection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLOS one. 16(5):e0251170.
Publisher | Google Scholor - Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, et al. (2020). COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 395(10229):1033-1034.
Publisher | Google Scholor - Morton CO, Griffiths JS, Loeffler J, Orr S, White PL. (2022). Defective antifungal immunity in patients with COVID-19. Front Immunol. 13:1080822.
Publisher | Google Scholor - Castro-Fuentes CA, Reyes-Montes MdR, Frias-De-León MG, Valencia-Ledezma OE, Acosta-Altamirano G, et al. (2022). Aspergillus-SARS-CoV-2 Coinfection: What Is Known? Pathogens. 11(11):1227.
Publisher | Google Scholor - Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. (2021). Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 384(8):693-704.
Publisher | Google Scholor - Latgé JP. (1999). Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 12(2):310-350.
Publisher | Google Scholor - Dimopoulos G, Almyroudi M-P, Myrianthefs P, Rello J. (2021). COVID-19-Associated Pulmonary Aspergillosis (CAPA). Journal of Intensive Medicine. 1(2):71-80.
Publisher | Google Scholor - Hoenigl M, Seidel D, Sprute R, Cunha C, Oliverio M, et al. (2022). COVID-19-associated fungal infections. Nat Microbiol. 7(8):1127-1140.
Publisher | Google Scholor - van Arkel ALE, Rijpstra TA, Belderbos HNA, van Wijngaarden P, Verweij PE, et al. (2020). COVID-19-associated Pulmonary Aspergillosis. Am J Respir Crit Care Med. 202(1):132-135.
Publisher | Google Scholor - Alanio A, Dellière S, Fodil S, Bretagne S, Mégarbane B. (2020). Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med. 8(6):48-49.
Publisher | Google Scholor - Bergmann F, Jorda A, Blaschke A, Gabler C, Bohdan S, et al. (2023). Pulmonary Aspergillosis in Critically Ill COVID-19 Patients Admitted to the Intensive Care Unit: A Retrospective Cohort Study. J Fungi. 9(3).
Publisher | Google Scholor - Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, et al. (2016). Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 62(4):1-50.
Publisher | Google Scholor - Ahmed N, Mahmood MS, Ullah MA, Araf Y, Rahaman TI, et al. (2022). COVID-19-Associated Candidiasis: Possible Patho-Mechanism, Predisposing Factors, and Prevention Strategies. Curr Microbiol. 79(5):127.
Publisher | Google Scholor - Tsai CS, Lee SS, Chen WC, Tseng CH, Lee NY, et al. (2022). COVID-19-associated candidiasis and the emerging concern of Candida auris infections. J Microbiol Immunol Infect.
Publisher | Google Scholor - Zhou F, Yu T, Du R, Fan G, Liu Y, et al. (2020). Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 395(10229):1054-1062.
Publisher | Google Scholor - Ripa M, Galli L, Poli A, Oltolini C, Spagnuolo V, et al. (2021). Secondary infections in patients hospitalized with COVID-19: incidence and predictive factors. Clin Microbiol Infect. 27(3):451-457.
Publisher | Google Scholor - Chowdhary A, Sharma C, Meis JF. (2017). Candida auris: A rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog. 13(5):e1006290.
Publisher | Google Scholor - Chandley P, Subba P, Rohatgi S. (2022). COVID-19-Associated Mucormycosis: A Matter of Concern Amid the SARS-CoV-2 Pandemic. Vaccines. 10(8).
Publisher | Google Scholor - Dam P, Cardoso MH, Mandal S, Franco OL, Sagiroglu P, et al. (2023). Surge of mucormycosis during the COVID-19 pandemic. Travel Med Infect Dis. 52:102557.
Publisher | Google Scholor - Patel A, Agarwal R, Rudramurthy SM, Shevkani M, Xess I, et al. (2021). Multicenter Epidemiologic Study of Coronavirus Disease-Associated Mucormycosis, India. Emerg Infect Dis. 27(9):2349-2359.
Publisher | Google Scholor - Kumar R, Misra AK, Dutta S, Gupta A, Kumar B, et al. (2022). A systematic review of mucormycosis cases in COVID-19: Is it an unholy trilogy of COVID-19, diabetes mellitus, and corticosteroids? J Family Med Prim Care. 11(6):2573-2580.
Publisher | Google Scholor - Pakdel F, Ahmadikia K, Salehi M, Tabari A, Jafari R, et al. (2021). Mucormycosis in patients with COVID-19: A cross-sectional descriptive multicenter study from Iran. Mycoses. 64(10):1238-1252.
Publisher | Google Scholor - Choudhary NK, Jain AK, Soni R, Gahlot N. (2021). Mucormycosis: A deadly black fungus infection among COVID-19 patients in India. Clinical Epidemiology and Global Health. 12:100900.
Publisher | Google Scholor - Regalla D, VanNatta M, Alam M, Malek AE. (2022). COVID-19-associated Cryptococcus infection (CACI): a review of literature and clinical pearls. Infection. 50(4):1007-1012.
Publisher | Google Scholor - Chastain DB, Kung VM, Vargas Barahona L, Jackson BT, Golpayegany S, et al. (2022). Characteristics and Outcomes of Cryptococcosis among Patients with and without COVID-19. J Fungi. 8(11).
Publisher | Google Scholor - Gentile I, Viceconte G, Lanzardo A, Zotta I, Zappulo E, et al. (2021). Pneumocystis jirovecii Pneumonia in Non-HIV Patients Recovering from COVID-19: A Single-Center Experience. Int J Environ Res Public Health. 18(21).
Publisher | Google Scholor - Cighir A, Mare AD, Cighir T, Coșeriu RL, Vintilă C, et al. (2023). Filamentous Fungi Infections: However, Another Victim of COVID-19? Life. 13(2).
Publisher | Google Scholor - Hoenigl M. (2021) Invasive Fungal Disease Complicating Coronavirus Disease 2019: When It Rains, It Spores. Clin Infect Dis. 73(7):1645-1648.
Publisher | Google Scholor - Raffaelli F, Tanzarella ES, De Pascale G, Tumbarello M. (2022). Invasive Respiratory Fungal Infections in COVID-19 Critically Ill Patients. J Fungi. 8(4).
Publisher | Google Scholor - Sasani E, Bahrami F, Salehi M, Aala F, Bakhtiari R, et al. (2023). Pneumocystis pneumonia in COVID-19 patients: A comprehensive review. Heliyon. 9(2):e13618.
Publisher | Google Scholor - White PL. (2021). Diagnosis of invasive fungal disease in coronavirus disease 2019: approaches and pitfalls. Curr Opin Infect Dis. 34(6):573-580.
Publisher | Google Scholor - Song G, Liang G, Liu W. (2020). Fungal Coinfections Associated with Global COVID-19 Pandemic: A Clinical and Diagnostic Perspective from China. Mycopathologia. 185(4):599-606.
Publisher | Google Scholor