Research Article
Intrathecal Opioids for Non-Emergent Cesarian Section In Latin American Women: A Retrospective Cohort Study (Re-Mice Study)
Anesthesia and Perioperative Medicine Department, Fundación Valle del Lili. Cali, Colombia
*Corresponding Author: Fredy Ariza,Anesthesia and Perioperative Medicine Department, Fundación Valle del Lili. Cali, Colombia
Citation: Fredy Ariza. (2023). Intrathecal Opioids for Non-Emergent Cesarian Section in Latin American Women: A Retrospective Cohort Study (Re-Mice Study). International Journal of Medical Case Reports and Reviews, BRS Publishers. 2(4); DOI: 10.59657/2837-8172.brs.23.024
Copyright: © 2023 Fredy Ariza, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: June 16, 2023 | Accepted: June 30, 2023 | Published: July 28, 2023
Abstract
Background: Common use of intrathecal opioids for cesarean sections has been limited due to reported adverse events, despite their significant role in postoperative analgesia. It has been suggested that a dose reduction could potentially reduce adverse effects without affecting efficacy of postoperative analgesia.
Objectives: The aim of this study was to identify the most frequently used intrathecal opioid doses by Latin-American women who underwent non-emergent cesarean sections at a reference center and reported adverse events during the first 6, 12, and 24 hours after administration.
Methods: We retrospectively analyzed a cohort of women that underwent non-emergent cesarean sections under spinal anesthesia during the period of January 2011 to June 2014. The associations between different doses of intrathecal morphine (≤50; 51-75; 76-100 and 101-150 mcg) with and without intrathecal fentanyl (20-25 mcg) and pruritus, postoperative nausea/vomiting, and respiratory depression probabilities were assessed using a uni-bivariate proportional hazards model and a multivariate logistic model.
Results: Out of 1,405 patients treated with any type of intrathecal opioid for cesarean section, 1,314 (93.5%) received intrathecal morphine. We detected a significant association between pruritus probability and intrathecal morphine dose during the first 6 postoperative hours. There was no correlation between postoperative nausea/vomiting occurrence and increasing intrathecal morphine doses.
Conclusions: Intrathecal morphine 50 to 150 mcg and intrathecal fentanyl 20 to 25 mcg are effective and safe. During the first 6 postoperative hours, pruritus, but not postoperative nausea/vomiting, shows a significant correlation with intrathecal morphine doses of 75 to 100 mcg.
Keywords: cesarean section; non-emergent; spinal; anesthesia; intrathecal; opioid; morphine
Introduction
Several studies have shown a dominant role for intrathecal opioids (ITOs) on intra- and postoperative analgesia for obstetric patients undergoing cesarean sections [1-5]. Unfortunately, related adverse events (nausea, vomiting, pruritus and ventilatory depression) have limited their use as a standard treatment during obstetric surgery [6-8]. Some authors have recommended a reduction in intrathecal morphine (ITM) dose as a strategy to reduce secondary effects without repercussions on efficacy and duration of analgesia [9-11]. Inter-individual differences in pain perception are limiting factors for the study of analgesic drugs. On the other hand, collateral effects are influenced by a myriad of sociocultural, biometrical and pharmacogenomic factors, as well as by drug efficacy and severity of surgical trauma [12-13].
Objectives
The aims of this study were to identify the most common ITO doses used by anesthesiologists at a level IV Latin-American Center for obstetric patients that undergo cesarean sections; and to analyze the frequency of related adverse events (pruritus, PONV, urinary retention, ventilatory depression and sedation) at 6, 12, and 24 postoperative hours and their potential association with ITO type and dose.
Materials And Methods
The RE-MICE (Retrospective study of Intrathecal Morphine in non-emergency Cesarean section) was a retrospective, observational cohort study conducted at a level IV Latin-American Center with IRB approval. It included all obstetric patients admitted for non-emergent cesarean sections and that received spinal anesthesia with or without ITOs between January 2011 and June-2014. Patients that received an epidural, a combined spinal-epidural, general anesthesia, or that had >10% missing data were excluded from the analysis. All patients were treated under a standardized institutional protocol that included preoperative clinical assessment and evaluation of fetal well-being before deciding on an anesthetic approach. All patients received a urinary catheter at the beginning of the procedure and a standardized analgesic plan that included a nonsteroidal anti-inflammatory medication (diclofenac, ketoprofen or ketorolac) after the third phase of delivery. After the procedure, the patients were moved to post anesthetic care unit (PACU) or the obstetric intensive care unit depending on the type of care needed and postoperative risk. Patients were continually monitored during PACU stay until total motor recovery was achieved before transfer to a hospital ward. Information about local anesthetic (LA) and ITO used for spinal anesthesia (type and dose), supplementary intraoperative analgesia, and use of intraoperative ondansetron were recorded for all included subjects. During the first 6, 12 and 24 postoperative hours, adverse events were recorded, including presence and intensity of pruritus, nausea, vomiting, urinary retention, sedation, and ventilatory depression. Pruritus was assessed based on intensity scores (1 = without pruritus; 2 = mild; 3 = moderate, treatment necessary; 4 = severe). Only scores greater than or equal to 3 were considered relevant. Ventilatory depression was defined as a respiratory rate less than 10 bpm, or a report of pulse oximetry less than 92%, independent of any type of intervention required. Postoperative sedation assessment was based on the Ramsay scale, and a score ≥ 3 was considered clinically relevant. Episodes of nausea or vomiting with or without intervention during any of the time intervals were reported. Information about pain perception and need for rescue analgesia was extracted from nurse charts if pain levels reached a score of ≥ 5 on a visual analogue scale.
Data management
Corresponding data were tabulated and verified for non-plausible information. To analyze exposition to ITM, patients were divided in four categories: those who did not receive ITM (ITM-0); and ITM doses of ≤50 (ITM-50), 51-75 (ITM-75), 76-100 (ITM-100), and 101-150 (ITM-150) µg. A univariate proportional hazards model and bivariate model were used to determine significant associations using STATA/SE 10.1 for Macintosh (College St, TX 77845 USA). A Fisher’s test (Student’s t-test) and Mann-Whitney test were used for parametrical and non-parametrical variables, respectively. Comparisons for dichotomic outcomes were performed using a chi-square test. Results are expressed as means (SD) or medians (interquartile ranges) for normal and non-normal distribution variables, respectively, and absolute numbers/proportions for dichotomic.
Results
During the study period, 1,761 cesareans were reported. 94.7% (n=1669), 1.2% (n=21) and 4% (n=71) of the surgeries were done under spinal, epidural, and general anesthesia, respectively. Seventy-two patients were excluded from analysis due to concomitant use of parenteral opioids or missing data. Out of 1,405 women receiving ITOs and LA, 1,314 received ITM with (24%) and without (65%) intrathecal fentanyl (ITF). Only 91 patients (6.5%) received ITF as a unique opioid added to LA (Figure 1)
Flow diagram of a retrospective analysis of a cohort of pregnant Latin-American women who underwent caesarian section under spinal anesthesia with and without intrathecal opioids.
Distribution of intrathecal morphine doses added to hyperbaric bupivacaine for a cohort of Latin American women that underwent cesarean section.
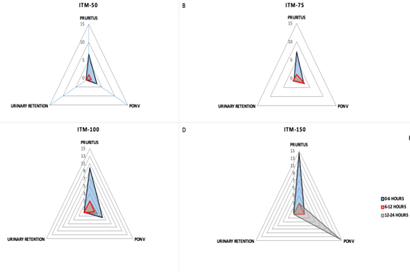
Risk for pruritus, urinary retention and PONV during first 6, 12 and 24 hours with four different dose ranges of intrathecal morphine in a cohort of Latin women.
| Event | ITO Dose | OR (95%CI) 6 hours | OR (95% CI) 12 hours | OR (95% CI) 24 hours |
| Pruritus | ITM-50 | 6.69 (1.40-31.87) | 1.00 (0.99-1.01) | 1.35 (0.16-11.26) |
| ITM-75 | 7.19 (1.66-31.14) | 1.016 (1-1.03) | 1.01 (1-1.02) | |
| ITM-100 | 9.87 (2.38-40.89) | 1.015 (1-1.02) | 1 (0.99-1.01) | |
| ITM-150 | 140.5 (6.32-3122) | 87.6 (5.27-1455) | 264.8 (14.79-4743) | |
| PONV | ITM-50 | 3.07 (1.12-8.48) | 1.21 (0.26-5.47) | 0.99 (0.98-1.00) |
| ITM-75 | 2.62 (1.04-6.59) | 3.18 (1.06-9.55) | 2.94 (0.62-13.96) | |
| ITM-100 | 3.74 (1.60-8.80) | 1.82 (0.61-5.40) | 0.75 (0.14-4.16) | |
| ITM-150 | 112.93 (6.72-1897) | 36.1 (2.22-587) | 140.5 (6.32-3122) | |
| Urinary Retention | ITM-50 | 1.01 (0.99-1.01) | 0.99 (0.99-1) | 0.99 (0.99-1) |
| ITM-75 | 0.99 (0.99-1) | 1.01 (0.99-1.01) | 1.45 (0.13-16.10) | |
| ITM-100 | 1.01 (1-1.01) | 1.01 (0.99-1) | 1.14 (0.11-10.99) | |
| ITM-150 | 264.8 (14.79-4743) | 530.6 (26.57-10598) | 0.99 (0.99-1) |
Table 1: Demographics and absolute distributions for ITM treatment by dose intervals.
Treatment with ITM results in a significant reduction of rescue analgesia at PACU during the first 6 postoperative hours compared to patients treated with LA alone. Concomitant use of ITF and ITM showed a slight reduction of rescue analgesia at PACU (no-ITF= 15.2% vs. ITF=11.27%; OR 0.71 (IC95% 0.5-0.99)), with no significant differences in the proportion of VAS>4, at 6 (7.51 vs. 5.43%), 12 (5.19 vs. 6.68%) and 24 postoperative hours (0.63 vs. 1.08%). Global incidence of pruritus at 6, 12 and 24 postoperative hours was 5%, 1.12% and 0.31%, respectively. We found a significant association between the probability of pruritus during the first 6 postoperative hours and ITM dose [ITM-0: 0.71%; ITM-50: 4.5%, OR 6.38% (IC95% 1.24-62.13); ITM-75: 4.9%, OR 6.86 (IC95% 1.62-61.12); ITM-100: 6.58%, OR 9.87 (IC95% 2.56-84.3); ITM-150: 50%, OR 14.1 (IC95% 4.69-85.2), p=0.002]. However, at 12 and 24 hours, pruritus incidence was reduced significantly, without significant differences between groups. Logistic regression analysis did not show any association between pruritus risk at 6, 12, or 24 hours and ITF use (p=0.64), or between pruritus risk and intraoperative use of ondansetron (p=0.59). The PONV rate for the group was higher during the first 6 postoperative hours (ITM 0: 2.13%; ITM 50:6.5%; ITM-75: 5.5%; ITM-100: 7.5% and ITM-150: 0%) when compared to 12 (1.41%, 1.70%, 2.41% and 0%, respectively) and 24 postoperative hours (0.71%, 0%, 2.05%, 0.7% and 50%, respectively). Multivariate regression analysis showed an association between ITO dose and PONV at 6 postoperative hours (coif 0.0006, t: 2.29; p= 0.022), but not at 12 or 24 hours. When performing multivariate regression analysis, there was no association between concomitant ITF use or PONV prophylaxis for this outcome. Almost all the patients (97%) used a urinary catheter for at least 12 hours. Six (0.37%), cases of urinary retention were observed at 6 postoperative hours (ITM-50 n=1; ITM-100 n=5). Three cases were detected at 12 postoperative hours (ITM-75 n=2 and ITM-100 n=1), and six at 24 hours (0,37%; MIT-0 n=1, MIT-75 n=2 MIT-100 n=3). The study did not detect any statistically significant differences among different groups regarding this item. Nonetheless, multivariate regression analysis showed a non-significant association between ITO dose and urinary retention (Coeff=0.0001, t=1.72; p=0.08). Global proportion of patients with mild to moderate sedation (Ramsay scale 3-4) was not statistically significant between groups at 6 postoperative hours (ITM 0: 0%; ITM-50: 1.14%; ITM-75: 0%; ITM-100: 0.13% and ITM-150:0%.) and 12 hours (0.71, 1.70, 1.13, 0 and 0%; respectively). Concomitant use of fentanyl was not associated with changes in the sedation scale for this cohort (p=0.67). Global incidence of respiratory depression within the first 24 hours was 1.87/1000 patients (3 episodes). There was one event of respiratory depression between 0 and 6 hours (ITM-100 group), 2 episodes between 6 and 12 postoperative hours (ITM-75 and ITM-100 groups), and no events between 12 and 24 hours. There were no significant correlations between ITM doses (p=0.54), or use of ITF alone or as an adjunct (p=0.51), and ventilatory depression.
Discussion
Ito use has become an important issue in obstetric analgesia, due to its impact on postoperative analgesia. However, its association with adverse effects has led to high dose variability. What may be adequate for a given ethnic group should not be generalized for others [12]. which is especially true for Latin-American women, where data is very scarce and there is evidence of pharmacogenomic differences within this subpopulation. This retrospective analysis during a 4-year period showed serious and non-serious AEs related to ITO use in obstetric patients. Although it was not the purpose of our study, we must highlight the impact of ITO use on postoperative analgesia for this obstetric cohort. ITM and ITF lessened the need for pain intervention in PACU and the pain perception of obstetric patients who underwent caesarian section during the first 6 postoperative hours. This intervention was effective for doses starting at 50 mcg. Our study found a global pruritus incidence similar to that reported for patients who did not receive ITOs (5%). We found a greater frequency of pruritus during the first 6 postoperative hours and a direct association with dose, especially for doses exceeding 75-100 mcg. Nonetheless, we found a significant reduction for this event between 6 and 24 postoperative hours, without differences between groups. We also observed that the addition of ITF at a dose of 20-25 mcg does not increase the incidence of this event. We believe low doses of ITM had an impact on the frequency of this adverse event in the studied groups. We hypothesize that our results can be generalized to other Latin American populations. Regarding respiratory depression, which is the most feared adverse event, high levels of progesterone in pregnant women have a potent activating effect on the respiratory center, which leads to a very low incidence of this adverse event, even lower than in the general population [14]. When different routes of opioid administration are compared (systemic vs. epidural vs. intrathecal), we found that incidence of respiratory depression is lower for the intrathecal route compared to the other ones (1.86%, 0.59% and 0.69%, respectively). In our study, the incidence of this AE was very low and we did not find any association with the administered doses. Urinary retention is another potential adverse event for intrathecal opioid use. A study by Kuiper’s et al. showed a diminished detrusor contractility, which is related to a reduction in the feeling of urinary urgency, which in turn leads to urinary retention [15]. The intensity and duration of this adverse event are proportionately related to the dose [16,17]. However, the reported incidence is very low. A study by Herrera et al. found a urinary retention incidence of 24%, following pruritus and PONV [14,18]. A study by Carvalho et al. compared the use of 50 mcg versus 100 mcg of ITM. They found that there were no statistically significant differences between the groups at different postoperative periods [11]. This is in accordance with our study, which found a significantly low incidence of urinary retention and that there were no differences between groups [19]. Interestingly, a study by Fares et al. found that ITM doses between 200 and 1000 μg granted an excellent analgesic effect and that there were no benefits with higher doses [20]. Our study suggests that low ITM doses could be used with minimal adverse events and good analgesic efficacy. Nonetheless, the use of low ITM doses could be explained by the concomitant use of other analgesic types, such as NSAIDs, as part of the multimodal pain management. The main limitations of our study are related to selection bias, since we only included surgeries under spinal anesthesia. We excluded any patients who underwent CS under general anesthesia (4%), who got an epidural catheter prior to the surgery (1.2%), or who used IV opioids (4%). If we had included them, they would have had a different incidence of adverse events or different requirements for analgesia. Another potential source of bias is the under-registration of adverse events by medical staffs. Mild adverse events, such as pruritus, could have been presented by patients but not recorded. There is scarce evidence for the efficiency and the safety of ITO use in Latin-American population. Thus, our study will provide valuable information about its safety profile, which is better than other analgesic techniques. Due to the large number of participants, this study could potentially provide demographic standards for our region.
Conclusion
We conclude that commonly used ITO doses for Latin-American women undergoing non-emergent cesarean section are lower than those usually reported for American and European countries. ITM doses between 50 and 150 mcg are related to low incidence of serious and non-serious AEs, most of which are usually seen during the first 6 postoperative hours. However, we found a significant increase in some of these AEs when doses exceeded 100 mcg. At commonly used doses of ITM, addition of ITF did not confer any changes in analgesia efficacy nor presentation of AEs for this cohort.
Acknowledgements
Dario Castaño MD, for his artwork contribution.
References
-
-->