Research Article
Impacts of Parquetina Nigrescens on Aluminum Chloride Induced Cognitive Impairment in Male Wistar Rats
- Abiola Asowata-Ayodele *
University of Medical Sciences, Department of Biosciences and Biotechnology, Ond, Nigeria.
*Corresponding Author: Abiola Asowata-Ayodele, University of Medical Sciences, Department of Biosciences and Biotechnology, Ond, Nigeria.
Citation: Abiola A. Ayodele. (2024). Impacts of Parquetina Nigrescens on Aluminum Chloride Induced Cognitive Impairment in Male Wistar Rats. Clinical Case Reports and Studies, BioRes Scientia Publishers. 6(4):1-11. DOI: 10.59657/2837-2565.brs.24.158
Copyright: © 2024 Abiola Asowata-Ayodele, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: July 16, 2024 | Accepted: July 30, 2024 | Published: August 06, 2024
Abstract
The incidence of cognitive impairment (CI) is associated with Alzheimer disease (AD). Currently available therapeutics strategies are mainly symptomatic, therefore there is an urgent need for drugs that are pharmacologically safe, cost effective and available with minimal side effects. The present study evaluated the neuroprotective potential of Parquetina nigrescens on Aluminium Chloride (AlCl3) induced male albino wistar rats, an AD model. Thirty male albino rats (160 g-240 g) were grouped in 1-5 (control, AlCl3 only, AlCl3 + Donepezil 5 mg/kg, AlCl3 + P. nigrescens 100mg/kg and AlCl3 + P. nigrescens 200 mg/kg). All treatments were carried out orally for 14 days. The behavioral test (Y-maze test and Novel Object Recognition Task) was assessed for learning and memory. The acetylcholinesterase (AChE) and some oxidative stress markers including MDA, CAT, SOD and GSH levels were carried out in the brain and serum. The histopathology analysis was carried out on the hippocampus and cortex. Exposure to AlCl3 significantly decreased the learning and memory in the Y-maze test and NORT. There was significant increase in the AChE activity and MDA level, while the levels of CAT, SOD and GSH were significantly decreased in the AlCl3 group. Aqueous treatment with P. nigrescens (100 mg/kg and 200 mg/kg) led to an inhibition in the AChE activity and MDA level, while there was an increase in the CAT, SOD and GSH levels. Histopathology results showed that there was increase in the GFAP in texpressionshe AlCl3 group. This study demonstrated that the aqueous extract of P. nigrescens exhibited neuroprotective effect against AlCl3 induced AD model.
Keywords: bitter guard; alzheimer’s disease; memory regulation; brain function
Introduction
Cognition known as the total mental process of acquiring knowledge and understanding through thought and memory regulation, in short it is the total functioning of the brain [1] the damage of this function is referred to cognitive impairment. Cognitive impairment (CI) is a highly complex central nervous system disorder which is characterized by a progressive reduction of cognitive function which can lead to poor motor coordination, confusion, loss of short-term or long-term memory and impaired judgment [1].
The common risk factor of cognitive impairment is characterized by an elevation of amyloid beta (Aβ) and phosphorylated Tau protein associated with alterations of the central cholinergic system and irreversible loss of cognitive function resulting to memory deterioration, neurotransmitter disturbances and aging which is the most common factor [1].
Aluminum chloride (AlCl3) is neurotoxic and has been proposed to be one of the environmental factors responsible for neurodegenerative disease like Alzheimer’s disorders [2]. Aluminum can easily cross blood brain barrier through specific high affinity receptors and upon entering leads to cognitive dysfunction by impairing cholinergic function and inducing oxidative stress [2]. In the brain aluminum mostly accumulates in most being in a frontal cortex and hippocampus portions [3]. Epidemiological and animal investigations revealed that the accretion of aluminum in the hippocampus results in the anomalous Aβ accumulation, neuro-inflammation, thereby leading to neuronal necrosis.
In addition, aluminum induces inflammatory responses, protein like neurofilaments, microtubules associated protein and Aβ of highly phosphorylated cytoskeletal implicated in Alzheimer’s disease [2]. Aluminum chloride has also shown to have negative effects on behavioral aspects like anxiety-related behavior in wistar rats [4].
Histology studies have revealed the neurodegenerative effects of aluminum in the cerebral cortex and hippocampus of rats especially at higher dose. The mechanism of AlCl3-inducted neurotoxicity and identification of effective treatment is an important public and occupational health priority for industrial and developing nations.
However, currently available therapeutic strategies are mainly symptomatic. The main treatments for the disease are acetylcholinesterase inhibitors and N-methyl-D-aspartate receptor antagonists [5]. Unfortunately, these drugs have been connected to several adverse effects like nausea, vomiting, anorexia and insomnia, due to non-selective action on a variety of organ tissues both centrally and peripherally [6].
The incidence of cognitive impairment is also associated with several serious and mental disease, such as Alzheimer’s disease, stroke or cerebrovascular diseases, and alcohol abuse. The mortality rate of cognitive impairment is 8 % and it tends to increase annually, with Alzheimer’s disease having one of the fastest annual progressions (20 %). Cognitive impairment has many negative impacts on health status, independence, and is also considered as a high-cost illness from a socio-economic perspective. Thus, a therapeutic approach preventing or curing cognitive impairment is crucial to maintain and even increase the health status of the global community.
There is an urgent need to continue investigation on natural products which can be used in the management of CI, medicinal plants are promising source of future drugs. Plants contain bioactive natural compounds with wide structural diversity that might match the therapeutic targets of Cognitive impairment and other neurological disorders. The long history of drug development from natural products proves that many natural compounds of plant origin have inspired the discovery of new drug entities or “lead compounds” [7].
Nowadays, drug discovery efforts are drawing major attention by focusing on the identification of bioactive compounds of plant origin, including those for cognitive function-enhancing drugs [8]. Fortunately, some studies have revealed several medicinal plants used in the management of CI, it includes Parquetina nigrescens, Azadirachta indica, Ocimum gratissimum Panax ginseng, Curcuma longa, Santalum album, Centella asiatica, Bacopa monniera and Zingiber officinale [9; 10]. In an ethnobotanical survey, Parquetina nigrescens was reported as one of the frequent plants used as memory enhancer locally [10]. Therefore, the selection in the choice of plant used for this study.
The plant has been used as an ingredient in the medications for insanity, as well as an aphrodisiac in East Africa, it’s also called bullock in equatorial West Africa or bitter guard in Nigeria, any part of the plant is useful for medicinal purposes [10]. As a constituent of a commercial herbal preparation (Jubi formular), P. nigrescens is used in the treatment of anemia in humans in Nigeria. Other uses include the decoction of the stem bark being given as cardiac tonic while the leaf and root decoctions have been used for the treatment of gonorrhea and menstrual disorders. The aim of this study was to determine the neurochemical impact of P. nigrescens on aluminum chloride induced cognitive impairment in male wistar rats.
Materials and Methods
Collection of plants
P. nigrescens were collected in the forest at Akure, Ondo State and was identified in the Department of Plant Biology and Biotechnology, University of Medical sciences, Ondo State and voucher number was collected from the Forest Research Institute (FRIN) Ibadan, Nigeria with voucher number FHI 107128.
Preparation of plant extract
The leaves of P. nigrescens were air dried for two weeks and then oven dried for 2 H at 40 oC. The leaves were grounded to form a powdery sample. 116 g of the powdered sample was weighed and extracted over 24 hours with 2000 ml of distilled water. The mixture was filtered using whatman filter paper and the filtrate was evaporated at 60 o C using a rotary evaporator. The filtrate was later transferred to a water bath to get the crude extract. The crude extract was stored in the freezer until needed. The total yield of the crude extract was 28 g.
Animals
Thirty male albino rats weighing 160 g-240 g were procured from the Animal house of the University of Medical Sciences, Ondo. Animals were maintained at 12/12 hour light/dark cycle at room temperature. Animals were acclimatized for fourteen days prior to the experiment. Animals had access to standard pellet diet and water. The experimental protocol was approved by the Animal Ethics Committee of the University of Medical Science, Ondo (attached ethical clearance).
Chemicals
Aluminum chloride was gotten from the Department of Chemistry of the University of Medical Sciences, Ondo. Donepezil hydrochloride was gotten from Uche care Pharmacy, Ondo.
Experimental design
The experimental rats were selected randomly and divided into five experimental groups, 6 rats each. The group 1 was kept as normal control and received distilled water orally. The group 2 was kept as the disease control and received aluminum chloride solution (150 mg/kg) for 14 days. The group 3 was pre-administered with the standard Donepezil hydrochloride (5 mg/kg) orally for 14 days and 1 hr before aluminum chloride administration in the last 7 days. Group 4 and 5 were also pre-administered with P. nigrescens extract at dose 100 and 200 mg/kg orally respectively for 14 days and 1 hr before aluminum chloride administration in the last 7 days.
Statistical Analysis
The data obtained was analysed using GraphPad Prism software version 7.0 and expressed as mean ± SEM. Statistical analysis was done using one-way ANOVA, followed by post-hoc test.
p-value less than 0.05 was considered statistically significant
Behavioral Assessment
Y-maze
This assessment was executed to inspect the functional and cognitive memory of investigational rats. The trial was executed in wooden equipment composed of 3 arms (75 cm in length, 15 cm in breadth and 10 cm in height) [11]. The position between every arm was 120°. 24 hrs thereafter, the rats were located in the middle of the equipment and permitted to move liberally for 5 min. the progression of arm movements was monitored via video recording. Afterward, the approaches of manifold arms and inclination of the animals toward entry a less recently visited arms were examined. The impulsive behavioral changes were determined via the following formula: 100 × (number of alterations/total arm entries-2).
Novel Object Recognition Test (NORT)
Exploration of novelty in an open-field has been extensively exploited in neuroscience studies of behavior and brain functions in rats and mice, and it is used in the study of memory. The apparatus consisted of a plywood box with modification (40 × 40 × 30 cm). The object to be discriminated was made of plywood in two different shapes of 8 cm height. On the day before test, mice were allowed to explore the box (without any object) for 2 min. On the day of test in the first trial (T1), two identical objects were presented in two opposite corners of the box, and the time taken by each mouse to explore the objects was recorded. Exploration was considered as directing the nose at a distance less than 2 cm to the object or touching with nose. During the second trial (T2, 24 h after T1), a new object replaced one of the objects presented in T1, and mice were left individually in the box for 5 min. The time spent for exploring the familiar (F) and the new (N) object was recorded separately, and discrimination index (DI) was calculated as N− F / N+ F. Care was taken to avoid place preference and the influence of olfactory stimuli by randomly changing the positions of the two objects during T2 and cleaning the apparatus with hydrogen peroxide. The first trial (T1) was conducted 60 min after the last treatment on the 14th day. The second trial (T2) was done 30 min after (T1).
Tissue Collection
All the animals used were anesthetized, each of the brain sample for biochemical analysis was kept in a 5 ml plain bottle containing 2 ml of tris KCl buffer pH 7.4 and cooled, while each brain sample for histopathology analysis was kept in a plain bottle containing formalin. With the help of disposable syringe, cardiac puncture was made to withdraw blood 24 hours after the behavioral studies. The blood was centrifuged and the serum was collected for biochemical analysis.
Biochemical Analysis
Determination of protein concentration
The protein concentrations of the various samples were determined by means of the Biuret method [11]. A slight modification: potassium iodide was added to the reagent to prevent precipitation of Cu2+ ions as cuprous oxide.
Procedure
The sample homogenate was diluted 10 times with distilled water. This was done to reduce the level of protein in the samples to the sensitivity range of Biuret method. 1ml of the diluted sample was taken and added to 3ml of Biuret reagent in triplicate. The mixture was incubated at room temperature for 30 minutes after which the absorbance was read at 540nm using distilled water as blank.
Determination of oxidative stress (LPO assessment)
Lipid peroxidation was determined by measuring the thiobarbituric acid reactive substances (TBARS) produced during lipid peroxidation [11]
Procedure
An aliquot of 0.4 ml of the sample was mixed with 1.6 ml of Tris-KCl buffer to which 0.5 ml of 30 % TCA was added. Then 0.5 ml of 0.75% TBA was added and placed in a water bath for 45 minutes at 80 oC. This was then cooled in ice and centrifuged at 3000 g for 15 minutes. The clear supernatant was collected and absorbance measured against a reference blank of distilled water at 532nm. The method of Adam-Vizi & Seregi was used to calculate the MDA levels [12]. Lipid peroxidation in units/mg protein or gram tissue was computed with a molar extinction coefficient of 1.56 x 105 M-1Cm-1.
MDA (units/mg protein) = 
Determination of Catalase activity
Claiborne method was used to determine the catalase activity [13].
Principle
The method is based on the loss of absorbance observed at 240 nm as catalase splits hydrogen peroxide. Despite the fact that hydrogen peroxide has no absorbance maximum at this wavelength, its absorbance correlates well enough with concentration to allow its use for a quantitative assay. Noble & Gibson coefficient of 0.0436 mM-1cm-1 was used [14]
Procedure
Hydrogen peroxide (2.95 ml of 19 mM solution) was pipetted into a 1 cm quartz cuvette and 50 µl of sample added. The mixture was rapidly inverted to mix and placed in a spectrophotometer. Change in absorbance was read at 240 nm every minute for 5 min.
Determination of Superoxide dismutase (SOD) activity
The method of Misra & Fridovich was used to determine the SOD activity [15].
Protocol
1 ml of sample was diluted in 9 ml of distilled water to make a 1 in 10 dilution. An aliquot of 0.2 ml of the diluted sample was added to 2.5ml of 0.05M carbonate buffer (pH 10.2) to equilibrate in the spectrophotometer and the reaction started by the addition of 0.3 ml of freshly prepared 0.3 mM adrenaline to the mixture which was quickly mixed by inversion. The reference cuvette contained 2.5ml buffer, 0.3ml of substrate (adrenaline) and 0.2ml of water. The increase in absorbance at 480nm was monitored every 30 seconds for 150 seconds.
Estimation of reduced Glutathione (GSH) level
The method of Beutler was followed in estimating the level of reduced glutathione (GSH) in liver supernatants [16].
Procedure
0.2ml of sample was added to 1.8ml of distilled water and 3ml of 4% Sulphursalicyclic acid was mixed with the sample. This was centrifuged at 3,000g for 4 minutes. Thereafter, 0.5ml of the supernatant was added to 4.5ml of Ellman reagent. A blank was prepared with 0.5ml of the diluted precipitating agent and 4ml of phosphate buffer and 0.5ml of Ellman’s reagent. The absorbance of the reaction mixture was read within 30 minutes of colour development at 412nm against a reagent blank.
Estimation of Acetylcholinesterase (AChE) activity
The Ellman’s method [18] was used to determine the acetylcholinesterase activity quantitatively. AChE activity was monitored at 412 nm in a mixture containing 100µL of 100mM phosphate buffer (ph 7.4), 5,5-dithiobis-(2-nitrobenzoic acid) (DTNB) (1.7mM), 15µL of the brain homogenate, and 1.4mM acetylthiocholine iodide. Enzyme activity was calculated and expressed as µmol AChE per min per mg of protein [18].
Histopathology
Immunohistochemistry of GFAP -1
The cortex was fixed in 10 % neutral buffered formalin, dehydrated and embedded in paraffin wax. Sections of 3 µm thickness were cut onto coated slides, de-parafinized and rehydrated. Heat-mediated antigen retrieval was performed using citrate-based antigen retrieval solution (pH 6.0), for 30 min. Sections were then treated using Mouse and Rabbit HRP/DAB IHC Detection kit. Endogenous peroxidase blocking (10 min) was performed. This was followed by incubation with the primary antibodies viz., mouse anti-GFAP (1:5000 dilution; 2 h 30 mins). Sections were then incubated in HRP (horse radishperoxidase) micro-Polymer Goat Anti-rabbit HRP (Abcam USA) for 30 min. The reaction was developed with DAB chromogen (Abcam, USA). Sections were then rinsed in water and counterstained with Mayer’s Haematoxylin, dehydrated, cleared and mounted with Dibutyl Phthalate Xylene (Dako).
Photomicrograph and Image Quantification
The processed tissues were viewed under a Digital Light microscope and digital photomicrographs were taken by an attached camera at x400 and x100 magnifications, using OMAX software. NIH sponsored ImageJ software was used for digital analysis of photomicrographs using the cell counter plugin. [19]
Results
Behavioural analysis
Brain
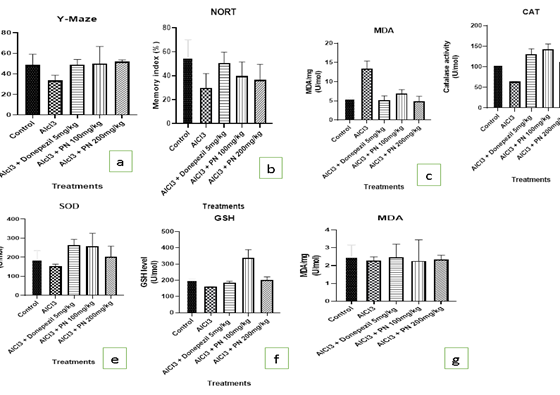
Figure: a- g
Serum and Cortex
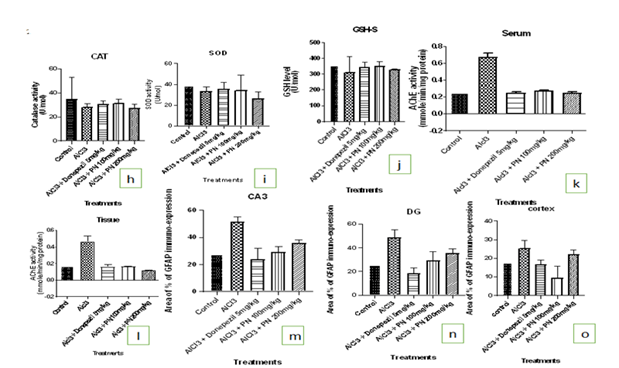
Figure: h-o
Y-maze: AlCl3 group showed significant decreased in the number of alterations compared to the control (p less than 0.05). The AlCl3 treated group with PN (100 mg/kg and 200 mg/kg) and donepezil (5 mg/kg) had a significant increase in the number of alterations compared to the AlCl3 group. (Figure a).
Novel Object Recognition Task
AlCl3 group showed significant decreased in learning and memory compared to the control (p less than 0.05). The AlCl3 treated group with PN (100 mg/kg and 200 mg/kg) and donepezil (5 mg/kg) had a significant increase in learning and memory compared to the AlCl3 group. (Figure b).
Biochemical analysis
Oxidative stress markers levels in the brain
The level of MDA was significantly increased in the brain of AlCl3 group compared to the control group. AlCl3 treated group with PN (100 mg/kg and 200 mg/kg) and donepezil (5 mg/kg) had a significant decrease in the MDA levels compared with the AlCl3 group (p less than 0.05).
The levels of the CAT, SOD and GSH was significantly decreased in the brain of the AlCl3 group compared to the control group (p < 0>
Acetylcholinesterase Enzyme Activity Test: The AChE activity in the brain and blood was signifcantly increased in the AlCl3 group when compared with the control group (p less than 0.05), while AlCl3 treated group with PN (100mg/kg and 200mg/kg) and donepezil (5mg/kg) significantly decreased the AChE activity when compared with the AlCl3 group (p less than 0.05). Figure c & 1d.
Histopathology Results
CA3
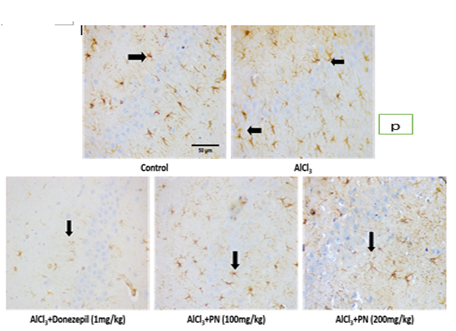
The control group showed expression of GFAP: The AlCl3 group showed increased expression of GFAP compared to the control group. Aqueous extract of P. nigrescens treated group showed decreased expression of GFAP at 100mg/kg and 200mg/kg. Treatment with Donepezil 5mg/kg also showed decreased expression of GFAP compared to other treatment groups.
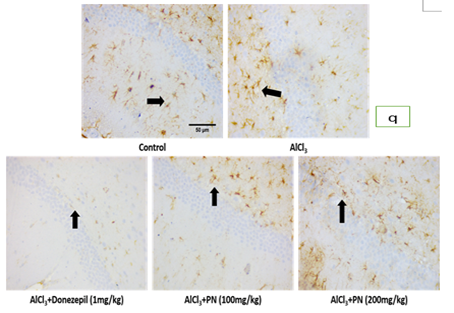
The control group showed expression of GFAP: The AlCl3 group showed increased expression of GFAP to the control group. Aqueous extract of P. nigrescens treated group showed decreased expression of GFAP at 100 mg/kg than 200 mg/kg. Treatment with Donepezil 5mg/kg also showed decreased expression of GFAP compared to other treatment groups.
The control group showed expression of GFAP: The AlCl3 group showed increased expression of GFAP compared to the control group. Aqueous extract of P. nigrescens treated group showed decreased expression of GFAP at 100 mg/kg than 200 mg/kg. Treatment with Donepezil 5mg/kg also showed decreased expression of GFAP compared to other treatment groups (Figure p-r).
Figure: p
Figure: q
Figure: r
Discussion
The current study investigated the behavioral, biochemical and histopathology alterations caused by AlCl3 exposure and the possible effect of treatment with the aqueous extract of P. nigrescens, a plant which has been reported as one of the frequent plants used as memory enhancer locally [10]. Memory function in animal models is generally assessed using various behavioral tasks including the Y-Maze test and the Novel Object Recognition Task (NORT). The Y-maze apparatus is used for the assessment of the short-term memory [20] and diminished percentage of alternation is an indicator of an impaired spatial working memory. This is because the rat cannot remember which arm it has just visited and thus shows decreased spontaneous alternation. Short-term memory impairment is the first clinical feature of AD and when the condition progresses, other cognitive functions such as the ability to analyze and usage of common objects and tools in human beings are impaired. This present study shows that AlCl3 group showed reduction in the number of arm entries and spontaneous alternation percentage compared to the control. This is consistent with a previous finding that showed a reduction of spontaneous alternation percentage in rats received AlCl3 [21, 22]. Moreover, AlCl3 treated rats significantly traveled less distance compared to other groups. It is well known that cerebellum is concerned with the locomotor activity [23], therefore, any disturbance in locomotion may indicate cerebellar impairment, which came in agreement with [24]. However, AlCl3 treated group P. nigrescens (100mg/kg and 200mg/kg) and Donepezil (5mg/kg) showed increased number of arm entries and spontaneous alternation percentage.
The second behavioral evaluation in the current study was the novel object recognition test. It is also a simple, fast and non-stressful test which is used to evaluate different aspects of memory in rodent. The test depends on the natural tendency of rodent to explore novel object [25], therefore it does not require long period of training days. The retention intervals may be short to detect the short-term memory or long to detect the long-term memory. This present study shows that AlCl3 group had lower discrimination and recognition values compared to the control. That could be due to impaired visual recognition memory reflecting the impairment of the anterior sub-hippocampal cortex as discussed by [26]. This is consistent with a previous finding that showed lower discrimination and recognition values of rats that received AlCl3 [27]. Although treatment with aqueous extract of P. nigrescens 100mg/kg showed higher discrimination and recognition value than P. nigrescens 200 mg/kg and with donepezil having highest value compared to the other groups.
Oxidative stress is an imbalance state between the generation and detoxification of reactive oxygen species (ROS) products. It could be assessed by measuring peroxidation products of ROS, such as lipid peroxidation, and antioxidant levels [27] Aluminum hastens lipid peroxidation and triggers augmented free radical accumulation, thus causing oxidative stress that ultimately leads to the neurotoxicity [27]. The brain is highly susceptible to the oxidative stress resulted from augmented status of free radicals and suppressed the antioxidant status subsequently toxicity [28]. The present study showed that in the brains of the AlCl3 group, the MDA level was significantly higher, suggesting that AlCl3 was able to induce high oxidative stress in rats. These results are in agreement with previous studies which reported Aluminum enhanced the level of MDA in the cortex hippocampus, cerebellum and in the whole brain. This present study also showed that exposure to aluminum inhibited the activity of CAT and SOD enzymes. The effect of aluminum exposure is consistent with the previous studies that decreased levels of CAT and SOD in the brain. Besides SOD and CAT, aluminum reduces the level of Glutathione (GSH), which is consistent with the previous reports [28]. However, treatment with aqueous extract of P. nigrescens decreased the MDA level and increases the levels of CAT, SOD and GSH in the brains of the AlCl3 treated group, which may be due to its antioxidant properties [29]. Though, there was no significant difference in the MDA, CAT, SOD and GSH level in the serum of the AlCl3 group compared to the control and the AlCl3 treated group.
Impaired cholinergic transmission is one of the factors implicated in the etiopathogenesis of memory deficit in AD. The neurodegeneration in frontal cortex and hippocampus areas of the brain [30] resulting in impaired cholinergic transmission occurs by two ways. Firstly, in AD patients, it occurs either due to the decline in acetylcholine (ACh) release or due to the decreased choline acetyltransferase activity, which results in the scarcity of Ach [30]. Secondly, elevated acetylcholinesterase (AChE) enzyme further adds to scarcity of ACh at synapse by degrading the available ACh. Thus, acetylcholine is considered as an important neurotransmitter for memory in brains, and preventing the decrease of the level of acetylcholine may be an effective treatment strategy for AD. Present study shows that activity of AChE in the brain and serum of AlCl3 group was significantly higher than that of the control group. This study is in agreement with previous studies which reported a significant rise AChE activity in the rat brain and serum after exposure to AlCl3 [31]. These may be as a result of Aluminum being a potent cholinotoxin which alters the blood-brain barrier, elevates AChE and decrease Ach transmission [31]. However, treatment with aqueous extract of P. nigrescens decreased the AChE activity in the brain and serum of the AlCl3 treated group. Thus P. nigrescens may have the tendency of having cholinesterase inhibiting properties for AD treatment.
Glial fibrillary acidic protein is a type III intermediate filament protein that maintains astrocyte mechanical strength, as well as the shape of cells. They serve as markers of astrocytic activity, and are usually much expressed during astrogliosis. Increased GFAP expression indicates astrogliosis and this rapid and severe activation augments/initiates an inflammatory response, which leads to brain injury and neuronal death [32]. Astrocytes play active roles in neuronal regulation and modulation. It has also been suggested that the loss of astrocyte functions may precede neurodegeneration and aluminum could be a contributing factor for this loss [33].This present study showed the Immunohistochemical reactivity of the temporal cortex, CA3 and Dentate gyrus (DG) of rats treated with 150 mg/kg body weight of AlCl3 revealed increased GFAP expression, which is in accordance with previous findings and may be related to a generic response of the central nervous system to neural injury [39]. Aqueous extract of P. nigrescens treated groups 100 mg/kg and 200 mg/kg showed less expression of GFAP, although treatment with Donepezil 5 mg/kg decreased GFAP expression compared to the other groups [39].
Several limitations and considerations
Animal Model Specificity: The study primarily uses animal models to simulate Alzheimer's disease (AD) conditions induced by AlCl3. While animal models are essential for preliminary research, findings may not always translate directly to human conditions due to biological differences.
Behavioral Tasks: The Y-Maze test and Novel Object Recognition Task are useful for assessing memory function in rodents. However, translating these results to human cognitive processes, especially for complex diseases like AD, requires cautious interpretation.
Biochemical and Histopathological Markers: While biochemical markers such as MDA (Malondialdehyde), CAT (Catalase), SOD (Superoxide dismutase), and GSH (Glutathione) levels provide insights into oxidative stress and antioxidant activity, their relevance to human neurodegenerative diseases needs validation
Generalization of Findings: The study focuses on specific biochemical pathways and behavioral outcomes in a controlled environment. Extrapolating these findings to broader clinical applications or different populations requires further clinical research.
Mechanistic Understanding: While the study suggests neuroprotective, antioxidative, and anti-inflammatory properties of P. nigrescens, the exact mechanisms through which these effects occur are not fully elucidated. Further mechanistic studies would strengthen the understanding of these effects.
The results of the study suggest that aqueous extract of the leaves of P. nigrescens possesses neuroprotective, antioxidative and anti-inflammatory properties by decreasing the MDA level and increasing CAT, SOD and GSH levels which improve memory and learning in the AD rat model. It also possesses cholinesterase inhibiting properties by decreasing AchE enzyme activity that destruct Ach which is excitatory neurotransmitter in the brain. These results represented satisfactory therapeutic approaches for intervention against the progressive neurological damage associated with AD, with special reference to oxidative insults. In conclusion, while the study provides promising insights into potential therapeutic interventions for AD-related cognitive decline using P. nigrescens extract, these findings warrant further investigation, including clinical trials to validate efficacy, safety, and optimal dosing in human subjects.
| S/N | Figure represented |
| A | Memory Function Determined by Y-Maze Test |
| B | Memory function determined by NORT |
| C | Effect of PN on the MDA level of AlCl3 induced rat’s brain. |
| D | Effect of PN on the CAT level of AlCl3 induced rat’s brain. |
| E | Effect of PN on the SOD level of AlCl3 induced rat’s brain. |
| F | Effect of PN on the GSH level of AlCl3 induced rat’s brain. |
| G | Effect of PN on the MDA level of AlCl3 induced rat’ serum. |
| H | Effect of PN on the CAT level of AlCl3 induced rat’s serum. |
| I | Effect of PN on the GSH level of AlCl3 induced rat’s serum |
| J | Effect of PN on the GSH level of AlCl3 induced rat’s serum |
| K | Effect of PN on the AChE activity in AlCl3 induced rat’s serum. |
| L | Effect of PN on the AChE activity in AlCl3 induced rat’s brain |
| M | The area % of GFAP immunoexpression in CA3 |
| N | The area % of GFAP immunoexpression in DG |
| O | The area % of GFAP immunoexpression in the cortex |
| P | Photomicrographs of immunohistochemical staining of GFAP in CA3 of hippocampi of all experimental groups |
| q | Photomicrographs of immunohistochemical staining of GFAP in DG of hippocampi of all experimental groups |
| r | Photomicrographs of immunohistochemical staining of GFAP in the cortex of all experimental groups |
References
- Helmi, H., Fakhrudin, N., Nurrochmad, A., & Ikawati, Z. (2021). Plant natural products for cognitive impairment: A review of the preclinical evidence. Journal of Applied Pharmaceutical Science, 11(6):1-14.
Publisher | Google Scholor - Anand, T., Pandareesh, M. D., Manu, T. M., Khanum, F., Roopa, N., Madhukar, N., & Nagaraju, N. (2017). Brahmi herbal drink mitigates aluminium chloride induced cognitive impairments. Defence Life Science Journal, 2(2):152-162.
Publisher | Google Scholor - Cheng, X. J., Gu, J. X., Pang, Y. P., Liu, J., Xu, T., Li, X. R., Hua, Y. Z., Newell, K. A., Huang, X. F., Yu, Y., & Liu, Y. (2019). Tacrine-hydrogen sulfide donor hybrid ameliorates cognitive impairment in the aluminum chloride mouse model of Alzheimer's disease. ACS Chemical Neuroscience, 10(8):3500-3509.
Publisher | Google Scholor - Sharma, D. R., Wani, W. Y., Sunkaria, A., Kandimalla, R. J., Verma, D., Cameotra, S. S., & Gill, K. D. (2013). Quercetin protects against chronic aluminum-induced oxidative stress and ensuing biochemical, cholinergic, and neurobehavioral impairments in rats. Neurotoxicity Research, 23(4):336-357.
Publisher | Google Scholor - Hafez, H. S., Ghareeb, D. A., & Saleh, S. R. (2017). Neuroprotective effect of ipriflavone against scopolamine-induced memory impairment in rats. Psychopharmacology, 234(20):3037-3053.
Publisher | Google Scholor - Mendiola-Precoma, J., Berumen, L. C., Padilla, K., & Garcia-Alcocer, G. (2016). Therapies for prevention and treatment of Alzheimer’s disease. Biomedical Research International, 2589276:17
Publisher | Google Scholor - Achilonu, M. C., & Dennis, O. U. (2015). Bioactive phytochemicals: Bioactivity, sources, preparations, and/or modifications via silver tetrafluoroborate mediation. Journal of Chemical Education, 92(12):1-22.
Publisher | Google Scholor - Benek, O., Jan, K., & Ondrej, S. A. (2020). Perspective on multi-target drugs for Alzheimer’s disease. Trends in Pharmacological Sciences, 41(7):434-445.
Publisher | Google Scholor - Thakur, A. K., Kamboj, P., & Goswami, K. (2018). Pathophysiology and management of Alzheimer’s disease: An overview. Journal of Analytical & Pharmaceutical Research, 9(2):226-235.
Publisher | Google Scholor - Babawale, O. P., Taiwo, F., & Adetunj, O. S. (2016). Ethnobotanical survey of plants used as memory enhancers in three states of southwestern Nigeria. Journal of Applied Pharmaceutical Science, 6(9):209-214.
Publisher | Google Scholor - Alawdi, S. H., El-Denshary, E. S., Safar, M. M., Eidi, H., David, M.-O., & Abdel-Wahhab, M. A. (2016). Neuroprotective effect of nanodiamond in Alzheimer’s disease rat model: A pivotal role for modulating NF-κB and STAT3 signaling. Molecular Neurobiology, 54(3):1906-1918.
Publisher | Google Scholor - Oluwole, F. S., Ayoade, J. T., & Busari, A. M. (2016). Gastroprotective mechanisms of imipramine on indomethacin-induced gastric ulcer in male Wistar rats. African Journal of Biomedical Research, 19:155-162.
Publisher | Google Scholor - Tedesco, S., Doyle, H., Redmond, G., & Sheehan, D. (2008). Gold nanoparticles and oxidative stress in Mytilus edulis. Marine Environmental Research, 66(1):131-133.
Publisher | Google Scholor - Lam, S. S., Martell, J. D., Kamer, K. J., Deerinck, T. J., Ellisman, M. H., et al. (2014). Directed evolution of APEX2 for electron microscopy and proximity labeling. Nature Methods, 12(1):51-54.
Publisher | Google Scholor - Kasapoglu, M., & Özben, T. (2001). Alterations of antioxidant enzymes and oxidative stress markers in aging. Experimental Gerontology, 36(2):209-220.
Publisher | Google Scholor - Chattopadhyay, R. R. (2003). Possible mechanism of hepatoprotective activity of Azadirachta indica leaf extract: Part II. Journal of Ethnopharmacology, 89(2-3):217-219.
Publisher | Google Scholor - Martínez, I., Herrera, A., Tames-Espinosa, M., Bondyale-Juez, D. R., Romero-Kutzner, V., et al. (2020). Protein in marine plankton: A comparison of spectrophotometric methods. Journal of Experimental Marine Biology and Ecology, 526:151357.
Publisher | Google Scholor - Erukainure, O. L., Ijomone, O. M., Oyebode, O. A., Chukwuma, C. I., Aschner, M., & Islam, M. S. (2019). Hyperglycemia-induced oxidative brain injury: Therapeutic effects of Cola nitida infusion against redox imbalance, cerebellar neuronal insults, and upregulated Nrf2 expression in type 2 diabetic rats. Food and Chemical Toxicology, 127:206-217.
Publisher | Google Scholor - Foyet, H. S., Hritcu, L., Ciobica, A., Stefan, M., Kamtchouing, P., & Cojocaru, D. (2011). Methanolic extract of Hibiscus asper leaves improves spatial memory deficits in the 6-hydroxydopamine-lesion rodent model of Parkinson’s disease. Journal of Ethnopharmacology, 133(2):773-779.
Publisher | Google Scholor - Khalifa, M., Safar, M. M., Abdelsalam, R. M., & Zaki, H. F. (2020). Telmisartan protects against aluminum-induced Alzheimer-like pathological changes in rats. Neurotoxicity Research, 37(2):275-285.
Publisher | Google Scholor - Ogunlade, B., Adelakun, S. A., & Agie, J. A. (2020). Nutritional supplementation of gallic acid ameliorates Alzheimer-type hippocampal neurodegeneration and cognitive impairment induced by aluminum chloride exposure in adult Wistar rats. Drug and Chemical Toxicology, 1-12.
Publisher | Google Scholor - Muzzu, T., Mitolo, S., Gava, G. P., & Schultz, S. R. (2018). Encoding of locomotion kinematics in the mouse cerebellum. PLoS ONE, 13(9):e0203900.
Publisher | Google Scholor - Hritcu, L., Noumedem, J. A., & Cioanca, O. (2014). Methanolic extract of Piper nigrum fruits improves memory impairment by decreasing brain oxidative stress in an amyloid beta (1–42) rat model of Alzheimer’s disease. Cellular and Molecular Neurobiology, 34:437-449.
Publisher | Google Scholor - Lueptow, L. M. (2017). Novel object recognition test for the investigation of learning and memory in mice. Journal of Visualized Experiments, 126:e55718.
Publisher | Google Scholor - Didic, M., Ranjeva, J.-P., Barbeau, E., Confort-Gouny, S., Le Fur, Y., et al. (2011). Impaired visual recognition memory in amnestic mild cognitive impairment is associated with mesiotemporal metabolic changes on magnetic resonance spectroscopic imaging. Journal of Alzheimer’s Disease, 22(4):1269-1279.
Publisher | Google Scholor - Ji, D., Wu, X., Li, D., Liu, P., Zhang, S., Gao, D., & Xiao, Y. (2020). Protective effects of chondroitin sulfate nano-selenium on a mouse model of Alzheimer’s disease. International Journal of Biological Macromolecules, 154:233-245.
Publisher | Google Scholor - Kawahara, M., & Kato-Negishi, M. (2011). Link between aluminum and the pathogenesis of Alzheimer’s disease: The integration of the aluminum and amyloid cascade hypotheses. International Journal of Alzheimer’s Disease, 276393:17.
Publisher | Google Scholor - Kumar, V., & Gill, K. D. (2014). Oxidative stress and mitochondrial dysfunction in aluminum neurotoxicity and its amelioration: A review. Neurotoxicology, 41:154-166.
Publisher | Google Scholor - Ajayi, L., Ayeleso, A., Oyedepo, T., & Mukwevho, E. (2021). Ameliorative potential of hydroethanolic leaf extract of Parquetinanigrescens on D-galactose-induced testicular injury. Molecules, 26(12):3424.
Publisher | Google Scholor - Nampoothiri, M., John, J., Kumar, N., Mudgal, J., Nampurath, G. K., et al. (2015). Modulatory role of simvastatin against aluminum chloride-induced behavioral and biochemical changes in rats. Behavioral Neurology, 1–9.
Publisher | Google Scholor - Stevanović, I. D., Jovanović, M. D., Jelenković, A., Čolić, M., Stojanović, I., & Ninković, M. (2009). Effects of L-NAME, a non-specific nitric oxide synthase inhibitor, on AlCl3-induced toxicity in the rat forebrain cortex. Journal of Veterinary Science, 10(1):15-21.
Publisher | Google Scholor - Ekong, M. B., Ekpo, M. M., Akpanyung, E. O., & Nwaokonko, D. U. (2017). Neuroprotective effect of Moringa oleifera leaf extract on aluminum-induced temporal cortical degeneration. Metabolic Brain Disease, 32(5):1437-1447.
Publisher | Google Scholor - Guo, G. W., & Liang, Y. X. (2001). Aluminum-induced apoptosis in cultured astrocytes and its effect on calcium homeostasis. Brain Research, 888(2):221-226.
Publisher | Google Scholor - Nedzvetsky, V. S., Tuzcu, M., & Yasar, A. (2006). Effects of vitamin E against aluminum neurotoxicity in rats. Biochemistry (Moscow):71(3):239-244.
Publisher | Google Scholor