Case Report
Hidden Threats Within: Unravelling Cardiac Amyloidosis in Rheumatoid Arthritis
- Miauro Cynthia Victoria *
Department of Neurosurgery, Dr. Rajendra Prasad Government Medical College (RPGMC), Himachal Pradesh, India.
*Corresponding Author: Miauro Cynthia Victoria, Department of Neurosurgery, Dr. Rajendra Prasad Government Medical College (RPGMC), Himachal Pradesh, India.
Citation: Miauro Cynthia Victoria. (2024). Hidden Threats Within: Unravelling Cardiac Amyloidosis in Rheumatoid Arthritis. Clinical Case Reports and Studies, BioRes Scientia Publishers. 5(6):1-4. DOI: 10.59657/2837-2565.brs.24.128
Copyright: © 2024 Miauro Cynthia Victoria, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: April 12, 2024 | Accepted: April 30, 2024 | Published: May 15, 2024
Abstract
Rheumatoid arthritis, an autoimmune condition, has widespread effects on various bodily systems, typically appearing as joint dysfunction. This persistent inflammation in the joints can lead to a gradual decline in a person's ability to carry out their daily activities. Additionally, the accumulation of serum amyloid A (AA), a type of acute-phase protein, in different organs can result in systemic AA amyloidosis, a rare but serious complication often associated with chronic inflammatory conditions like rheumatoid arthritis (RA). In AA amyloidosis, the kidneys and gastrointestinal tract are the most commonly affected organs, while cardiac involvement occurs in roughly 10% of cases. Cardiac amyloidosis presents as restrictive cardiomyopathy, heart failure, and conduction disorders, ultimately leading to a reduced quality of life and, in severe cases, death. This disease is classified into two subtypes: transthyretin cardiac amyloidosis (ATTR-CA) and immunoglobulin light chain cardiac amyloidosis (AL- CA), based on the specific precursor protein that forms amyloid deposits within the heart muscle. Rheumatologists should remain vigilant for the development of AA amyloidosis in RA patients to enable early intervention.
Keywords: ATTRwt; cardiology; rheumatoid arthritis
Introduction
Rheumatoid arthritis, an autoimmune condition, has widespread effects on various bodily systems, typically appearing as joint dysfunction. This persistent inflammation in the joints can lead to a gradual decline in a person's ability to carry out their daily activities. Additionally, the accumulation of serum amyloid A (AA) [1,2], a type of acute-phase protein, in different organs can result in systemic AA amyloidosis, a rare but serious complication often associated with chronic inflammatory conditions like rheumatoid arthritis (RA) [3]. In AA amyloidosis, the kidneys and gastrointestinal tract are the most commonly affected organs, while cardiac involvement occurs in roughly 10% of cases [4]. Cardiac amyloidosis presents as restrictive cardiomyopathy, heart failure, and conduction disorders, ultimately leading to a reduced quality of life and, in severe cases, death [1]. This disease is classified into two subtypes: transthyretin cardiac amyloidosis (ATTR-CA) and immunoglobulin light chain cardiac amyloidosis (AL- CA), based on the specific precursor protein that forms amyloid deposits within the heart muscle [5]. Rheumatologists should remain vigilant for the development of AA amyloidosis in RA patients to enable early intervention. Conversely, wildtype TTR amyloidosis (ATTRwt) primarily affects elderly individuals and predominantly targets the heart, tendons, and ligaments. Symptoms of this condition may include arrhythmias, heart failure, carpal tunnel syndrome, and spinal stenosis [6,7]. This case report involves an elderly patient with RA who presented with dizziness and was subsequently diagnosed with systemic ATTRwt amyloidosis. Ventricular tachycardia arrhythmia was detected through ECG holter monitoring, and echocardiography revealed specific patterns of amyloid deposits with apical sparing. A bone scan imaging procedure confirmed the presence of amyloid deposits.
Case Report
We are presenting the case of a 74-year-old male patient presenting to the outpatient cardiology clinic for dizziness and faint-like symptoms. Patient’s past medical history includes diabetes mellitus on medical therapy and rheumatoid arthritis diagnosed in 2016 (recently on Ebetrexate and Prednisone). Upon presentation, vital signs were stable and within normal range. Physical exam showed nontoxic general appearance, normal neurologic examination, clear lung auscultation, regular heart sounds with no added murmur, and 2+ peripheral pulses. Review of system revealed chronic shoulder and ankle tendinopathy, as well as the recent sensation of episodes of dizziness associated with fatigue and pre-syncopy. Patient denied any syncopal episodes. Baseline electrocardiogram (ECG) showed normal sinus rhythm, normal QRS complex, normal axis and no repolarization or ST segment changes. Echocardiogram showed moderate concentric left ventricular hypertrophy with normal systolic and diastolic left and right ventricular function, normal valves structure and function but severely increased apical strain that compensates a concentric diffuse attenuation (figure1,2).
Figure 1: Parasternal long axis view shows concentric left ventricular hypertrophy marked by the straight yellow line on interventricular septum and posterior wall.
Figure 2: Global longitudinal strain of the left ventricle showed severely reduced longitudinal strain of all ventricle segments (less than -18%) marked by light red on the image with apical segment sparing marked by dark red
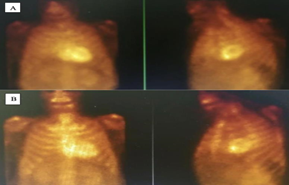
The decision was made to do a 24-hour ECG Holtor monitoring and basic laboratory tests including serum/urine protein electrophoresis and light chains. All laboratory workup came back normal. Holter ECG showed polymorphic premature complexes with one episode of non-sustained ventricular tachycardia correlated clinically with the sensation of dizziness (figure 3). Due to the combined result of Echocardiography and ECG Holtor monitoring, bone scintigraphy was ordered to rule out TTR amyloidosis (figure 4A, and 4B).
Figure 3: Non sustained ventricular tachycardia marked red color preceded by normal rhythm and premature ventricular beats in couplets marked by the blue arrow
Figure 4: Bone scintigraphy showed prominent tracer uptake diffusely observed at myocardium: visual grade 3 for cardiac amyloidosis assessed at both 1st h (top) and 3rd h(bottom) Figure 4A: visual grade 3 for cardiac amyloidosis assessed at 1 hour. Figure 4B: visual grade 3 for cardiac amyloidosis assessed at 3 hours.
The patient was diagnosed with TTR amyloidosis by the bone scintigraphy. His rheumatologist had planned to stop ebetrexate and prednisone for the clinical and biologic improvement. We contacted the rheumatologist to revisit the old medical chart: History goes back to 2016 when patient visit the rheumatologist for chronic tendinopathy mainly the right 2nd and 3rd digit and left shoulder pain. Laboratories at that time showed normal inflammatory markers. Ultrasound of right hand showed chronic digit synovitis. Inflammatory markers were checked annually and were normal except in 2019 when rheumatoid factor (RF) was 16 IU/ml (normal range less than 15 IU/ml). He was started on ebetrexate and prednisolone with improvement in joints pain.
Discussion
Amyloidosis is a broad term used to describe a group of diseases characterized by the buildup of insoluble amyloid fibrils in organs or tissues, leading to dysfunction of these organs and eventually resulting in death [8]. Amyloid fibrils form from soluble precursor proteins, and the number of known amyloidogenic proteins has increased over time [8]. The four most common systemic types of amyloidosis are light chain (AL), AA, β2-microglobulin (β2m), and wild-type/mutated TTR [9]. According to the First Nationwide Survey of 199 Patients with Amyloid A Amyloidosis in Japan, clinical symptoms at the time of AAA diagnosis included moderate to severe renal dysfunction (46.2%), significant proteinuria (30.7%), intractable diarrhea (32.2%), melena (4.5%), paralytic ileus (3.5%), heart failure (11.6%), cardiac conduction disturbances (10.1%), arrhythmia (5.5%), and hypothyroidism (11.6%) [7].
We present a case of a patient who was diagnosed with rheumatoid arthritis based on clinical symptoms, even though their laboratory tests were negative. The patient sought further evaluation due to episodes of dizziness and presyncope. Given the clinical presentation, we decided to conduct a 24-hour cardiac ECG Holter monitoring to detect any underlying arrhythmias responsible for the patient's symptoms. The Holter monitoring revealed an episode of ventricular tachycardia that coincided with the patient's reported dizziness. A recent study by Vitarelli et al. identified characteristic features of the amyloid heart, including reduced basal strain and regional variations in longitudinal strain from the base to the apex. They found that a relative "apical sparing" pattern in longitudinal strain is a distinctive and reliable way to differentiate cardiac amyloidosis from other causes of left ventricular hypertrophy [10]. In our patient, we performed an
echocardiogram assessment and observed a typical apical sparing pattern in global longitudinal strain (GLS). Recently, bone nuclear scintigraphy has emerged as a noninvasive diagnostic test with high sensitivity (92%) and specificity (95%) for ATTR-CA, especially in the absence of monoclonal gammopathy. This test uses a nuclear radiotracer, such as 99mTc-PYP in the United States or 99mTc-DPD/99mTc-HMDP elsewhere [4]. Cardiac bone scanning can provide additional diagnostic accuracy and grading. Grades range from 0 to 3, with grades 2 and 3 being diagnostic of ATTR-CA [5]. In our case, the diagnosis of ATTRwt (wild-type) amyloidosis was confirmed based on the cardiac bone scan. ATTRwt primarily affects the heart, tendons, ligaments, kidneys, thyroid, peripheral nerves, and lungs. Symptoms often involve the joints, ligaments, and heart, and affected patients may experience carpal tunnel syndrome, spinal stenosis, cardiac hypertrophy, arrhythmia, and heart failure [6,7]. Tsukada et al. suggested that ATTRwt may also lead to arthritis, although synovial biopsy was not performed in our patient to definitively establish the cause of the arthritis [11]. Considering the patient's negative rheumatoid factor test, polyarthritis affecting the hands, shoulders, and elbows, synovial thickening, and poor response to disease- modifying anti-rheumatic drugs, there is a suspicion that amyloidosis may be the underlying cause of the joint symptoms. Previous reports indicate that various types of amyloids can lead to severe joint problems, known as amyloid arthropathy [12]. Sekijima et al. found that in a case study of 51 patients with wild-type ATTR amyloidosis, the frequency of cardiac failure was 69%, while cardiac conduction defects/arrhythmias were present in 22% of initial presentations. However, only 2% of these patients initially exhibited arthralgia related to wild-type ATTR amyloidosis. This suggests that not all initial symptoms associated with wild-type ATTR amyloidosis involve the heart [13].
Conclusion
In conclusion, we suggest that our patient was falsly diagnosed with RA which is why he didn’t respond to medical therapy. Tafamidis meglumine is effective and it was approved for the treatment of cardiomyopathy with both hereditary ATTR amyloidosis and wild-type ATTR amyloidosis, as well as those with polyneuropathy and hereditary ATTR amyloidosis. Since our patient have symptoms of cardiac arrhythmias with proved apical sparing on GLS echocardiography, so he is allowed to be treated with Tafamidis meglumine. Treating him with Tafamidis meglumine will be the next step.
References
- Nakamura T. (2008). Clinical strategies for amyloid A amyloidosis secondary to rheumatoid arthritis. Modern rheumatology, 18(2):109-118.
Publisher | Google Scholor - Lachmann HJ, Goodman HJ, Gilbertson JA, et al. (2007). Natural history and outcome in systemic AA amyloidosis. The New England journal of medicine, 356(23):2361-2371.
Publisher | Google Scholor - Nakamura T. (2011). Amyloid A amyloidosis secondary to rheumatoid arthritis: development of pathophysiology and treatments. ClinExp Rheumatol. 29:850-857.
Publisher | Google Scholor - Okuda Y, Yamada T, Ueda M, et al. (2018). First Nationwide Survey of 199 Patients with Amyloid an Amyloidosis in Japan. Internal medicine, 57(23):3351-3355.
Publisher | Google Scholor - Jonah Rubin and Mathew S. Maurer. (2020). Cardiac Amyloidosis: Overlooked, Underappreciated, and Treatable. Annu. Rev.Med., 71:203-219
Publisher | Google Scholor - Sueyoshi T, Ueda M, Jono H, et al. (2011). Wild-type transthyretin-derived amyloidosis in various ligaments and tendons. Human pathology, 42(9):1259-1264.
Publisher | Google Scholor - Sekijima Y, Yazaki M, Ueda M, et al. (2018). First nationwide survey on systemic wild- type ATTR amyloidosis in Japan. Amyloid : the international journal of experimental and clinical investigation. The official journal of the International Society of Amyloidosis, 25(1):8-10.
Publisher | Google Scholor - Brendan Wisniowski, Ashutosh Wechalekar. (2020). Confirming the Diagnosis of Amyloidosis. Acta Haematol, 143:312-321
Publisher | Google Scholor - Nuvolone M, Merlini G. (2017). Emerging therapeutic targets currently under investigation for the treatment of systemic amyloidosis. Expert opinion on therapeutic targets, 21(12):1095-1110.
Publisher | Google Scholor - Vitarelli A, Lai S, Petrucci MT, et al. (2018). Biventricular assessment of lightchain amyloidosis using 3D speckle tracking echocardiography: differentiation from other forms of myocardial hypertrophy. Int J Cardiol, 271:371-377.
Publisher | Google Scholor - Tsukada T, Tanaka M, Miyazaki Y, et al. (2020). A case of unilateral shoulder joint hydrarthrosis with wild-type amyloidogenic transthyretin amyloidosis. Modern rheumatology case reports, 4(2):312-317.
Publisher | Google Scholor - Toshiaki Tsukadaa, Masamitsu Tanakab, Yoichi Miyazakib, Yoshihiro Nishiurac, Taro Yamashitad et al. (2020). A case of unilateral shoulder joint hydrarthrosis with wild- type amyloidogenic transthyretin amyloidosis. Modern Rheumatology Case Reports.
Publisher | Google Scholor - Rigopoulos AG, Ali M, Abate E, et al. Advances in the diagnosis and treatment of transthyretin amyloidosis with cardiac involvement. Heart Fail Rev.2019;24(4):521-533.
Publisher | Google Scholor