Research Article
Evaluation of Cytokines in Tears, Saliva and Serum of Patients with Sjogren’s Disease at Different Stages of Disease
1.Department of Medicine, SUNY at Buffalo School of Medicine, Ellicott Street Buffalo, New York, United States.
2.Scheie Eye Institute, University of Pennsylvania, Philadelphia, Pennsylvania, United States.
*Corresponding Author: Julian L. Ambrus Jr, Department of Medicine, SUNY at Buffalo School of Medicine, Ellicott Street Buffalo, New York, United States.
Citation: A. Jacob, V.Y. Bunya, A. Jamil, Julian L. Ambrus Jr. (2023). Evaluation of Cytokines in Tears, Saliva and Serum of Patients with Sjogren’s Disease at Different Stages of Disease. Journal of Clinical Rheumatology and Arthritis, BioRes Scientia Publishers. 1(2):1-11. DOI: 10.59657/2993-6977.brs.23.010
Copyright: © 2023 Julian L. Ambrus Jr, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: December 08, 2023 | Accepted: December 23, 2023 | Published: December 29, 2023
Abstract
Sjogren’s disease (SjD) is a common autoimmune disease that causes significant morbidity. Patients are often identified late in the course of the disease making the potential for tissue recovery limited. At the same time, the nature of disease in different organs, i.e., salivary glands, lacrimal glands, lungs and other organs, has been shown to be variable. The current studies were undertaken to evaluate cytokine markers for disease activity in different tissues, i.e., tears, saliva and serum, at different stages of disease along with serum autoantibody markers at different stages of disease to identify sensitive methods for diagnosis and potentially novel approaches to therapy.
We found that cytokines were identified more readily in tears than in saliva or serum. Elevations in IL-6 in tears were characteristic of patients with early SjD while elevations of IL-6, TNF-a and lymphotoxin were noted in patients with long-standing SjD. In serum, TNF-a and IL-2were elevated only in patients with long-standing SjD. In saliva, there were no statistically significant changes for any of the cytokines tested. Thus, different cytokines characterize SjD in early versus long-standing disease.
With regards to autoantibodies, SP1, CA6 and PSP were more sensitive markers for clinically defined SjD than SSA. Thus, these studies suggest a need for using an expanded list of cytokine and autoantibody markers to define SjD as well as evaluation of cytokines in tears, saliva and serum to help define the stage of the disease and potentially determine the nature of therapy that would be appropriate for individual patients.
Keywords: cytokines; saliva; serum; patient; sjogren’s disease; common autoimmune disease
Introduction
Sjogren’s disease (SjD) is a common autoimmune disease that includes xerostomia and xerophthalmia in all patients but can variably include other disease manifestations in individual patients including lung disease, kidney disease, neurologic disease and lymphoma [1]. Various cytokines have been implicated as drivers of disease, especially type 1 interferon, but cytokine inhibition has not been shown to be a successful treatment strategy [2-4]. Furthermore, patients with SjD are often identified after they have had disease for 1-2 years so permanent damage to the salivary and lacrimal glands can occur during that time. Current diagnostic criteria are not good at identifying patients early in the course of their disease [5, 6]. Recent studies have also identified the fact that the mechanism of injury to the lacrimal glands is not identical to the mechanisms of the salivary glands, and in fact there can be differences in the involvement of the submandibular and parotid glands [7-9].
We undertook the current studies to evaluate the cytokines in tears, saliva and serum of patients with disease less than 2 years compared to those with SjD for greater than 5 years. We set out to determine whether there were differences in the various tissues and different time points that would suggest specific forms of therapy in individual patients, depending upon the stage of their disease and perhaps the nature of their disease, which likely differs among individual patients.
Materials and Methods
Patients
Patients were recruited from the Immunology clinics at SUNY at Buffalo and the Ophthalmology clinics at the University of Pennsylvania. Normal controls were both age and sex matched friends of patients or independently recruited from SUNY at Buffalo staff and friends. The diagnosis of SjD was based upon clinical symptoms, Schirmer testing, measurement of saliva production and autoantibodies. Interestingly, of 23 recruited “normal”, 10 had reduced tear and saliva production consistent with SjD. Ultimately the studies included 5 normal (4 males and 1 female), 5 patients with early Sjogren’s (< 2> 5 years; all female). There were many subjects evaluated who could not be unambiguously assigned to a particular group and were therefore not included in this analysis.
Sample collection and Preparation
Saliva, serum and tear samples were collected before eating/drinking (except water) and brushing teeth in a small window of time: the first subject at 9 AM and the last subject at 10.30 AM on a given day. This was done to: a) minimize the diurnal variation of the cytokines and b) reduce the inconvenience to the subjects. The order of collection was: 1) saliva, 2) tears and 3) serum.
Saliva was collected using the saliva collection aid (Sali metrics, LLC; Carlsbad, CA), according to the manufacturer’s instructions. The time of collection was dependent on the volume of saliva produced. The goal was to collect slightly more than 1 ml, if possible. So, the time varied from less than 5 min to 20 min. The saliva was kept on ice immediately after collection and the required volume of protease inhibitor cocktail was added (complete Protease Inhibitor Cocktail; Roche). The saliva samples were centrifuged at 15,000 × g for 15 min at 4°C to eliminate insoluble material. The samples were divided into 0.5 mL aliquots and stored at -800C.
Tears were collected using Schirmer strips (Eye Care and Cure, Tucson, AZ). The strip was placed between the eye and the lower eyelid in the absence of anesthesia and the patient closed their eyelids for the 5- minute time period during which the tears were collected. The strips were immediately placed in 2 ml microcentrifuge tubes on dry ice until storage at -80o C. Elution was done from the strip using Milliplex buffer by incubating at room temperature for 5 min, followed by centrifugation at 14,000 x g for 1.5 min at 4oC as previously described [10]. The eluted tear samples were stored at -800C.
Serum was collected by phlebotomy. A red-top tube without anticoagulant was used for serum collection. The tube was placed upright in a test tube rack in darkness at room temperature for 45 min to allow a clot to form. Serum was separated from the clot by centrifugation at 1500 X g for 15 minutes at 4°C. The serum was removed and divided into 0.5 mL aliquots and stored at -800C.
Measurement of cytokines
Cytokine levels in serum, saliva and tears were determined by Assay Gate, Inc (Ijamsville, MD) using MSD-ECL assay (11), which is an electrochemiluminescence assay developed by proprietary technology of MSD (Meso Scale Discovery). Electro chemiluminescent labels (called SULFO-TAG) are conjugated to detection antibodies, and they emit light when stimulated by electricity. Light intensity is then measured to quantify analytes in the sample. For serum and saliva, levels of IL-2, IL-6, IL-17, TNF-a, TNF-b (lymphotoxin–LT), interferon–gamma (IFN-g) and interferon-alpha (IFN-a) were determined, while for tears levels of IL-6, IL-17, TNF-a, TNF-b and IFN-g were determined.
Autoantibodies
Measurement or SSA, SSB, anti-SP1, anti – CA6 and anti-PSP were performed from sera using an ELISA assay as previously described (12). Briefly, 96-well plats are coated with purified recombinant antigen. After blocking, the wells are treated with serum and the bound antibodies are detected with anti-human antibodies (IgG, IgM or IgA) conjugated to Horse Radish Peroxidase using TMB substrate.
Receiver-Operating Characteristic (ROC) Analysis
ROC analysis was performed as previously described (13). An ROC curve is a plot of true positive rate on the y axis against false positive rate on the x axis. AUC (Area Under the Curve) of an ROC curve gives an estimate of diagnostic accuracy. AUC of 0.5 denotes random chance and AUC of 1 denotes perfect accuracy.
Results
Patients were defined as having SjD based on clinical symptoms, salivary flow rates and Schirmer’s tests. They met clinical criteria for SJD, based on clinical criteria of the American College of Rheumatology (14). Table 1 shows the salivary flow rates and Schirmer test values for the various groups. Tear production was reduced in both early and long-standing SjD, while saliva was reduced significantly in long-standing SjD, but minimally in early SjD. Collection was done for longer than 5 minutes in some patients to get enough volume for the cytokine analysis. Measurement of autoantibodies in the study patients revealed SSA or SSB antibodies in none of the normal controls, 1 early SjD patient (SSB) and 1 late SjD patient (SSA). Antibodies to SP1, CA6 or PSP were found in 3 normal controls, 4 early SS patients and 4 long-standing SS patients; the antibody titer in late SS patients was much higher. Therefore SSA, which is the antibody in the diagnostic criteria for SjD, was only in 10% of total SS patients [14], while antibodies to SP1, CA6 and PSP were in 80% of all SjD patients diagnosed from clinical criteria and 80% of all late SS patients. One normal control had CA6 antibodies, and two had PSP antibodies. We do not know whether this suggests that they will get SjD in the future [15].
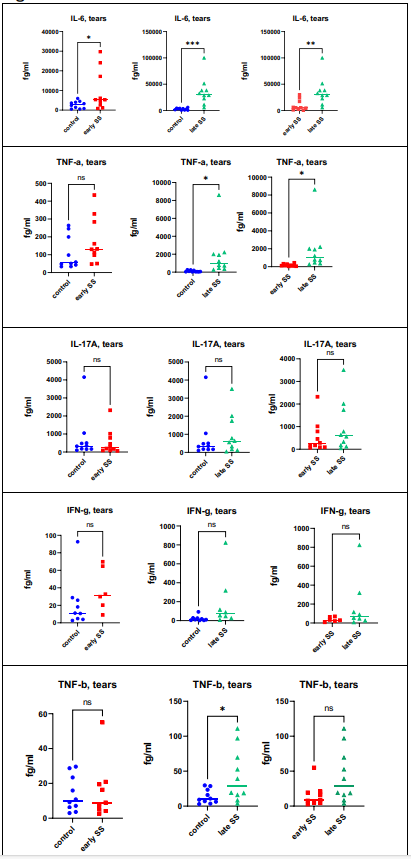
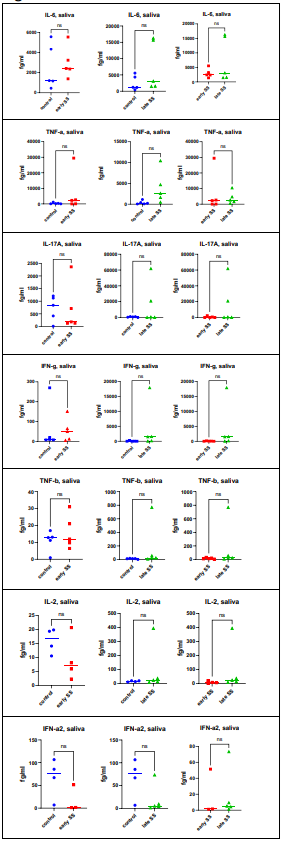
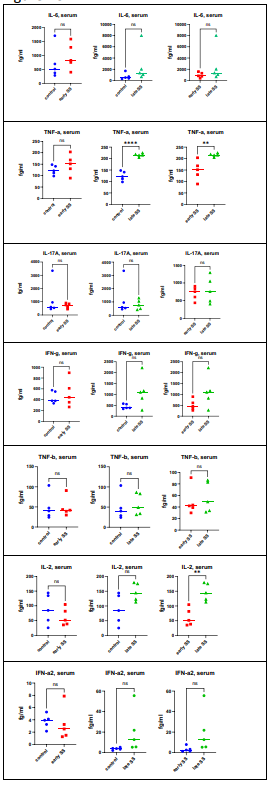
Measurement of cytokines was performed in tears, saliva and serum. Figure 1A demonstrates the findings for the quantitation of IL-6, IL-17, TNF-a, TNF-b (lymphotoxin – LT) and interferon – gamma (IFN-g) in tears. When comparing normal controls with patients with early SjD, only 1 cytokine showed statistically significant differences: IL-6 (p = .0482) was increased in SjD patients compared to normal controls. When comparing controls with patients with late SjD, IL-6 (p = .0010) TNF-a (p = .0405) and LT (p = .0286) were increased in SS patients. The differences between early SjD versus late SjhD were significant for IL-6 (p = .0098) and TNF-a (p = .0484). When evaluating saliva (Figure 1B), there were no statistically significant differences between the different groups. When evaluating sera (Figure 1C), the statistically significant findings were seen in controls versus late SjD participants: TNF -a (p =< 0 p =.0021) p =.>
Figure 1(A): Cytokines were measured in Tears as described in Materials and Methods. When comparing normal controls with patients with early SS, only 1 cytokine showed statistically significant differences: IL-6 (p=.0482) was increased in SS patients compared to normal controls. When comparing controls with patients with late SS, IL-6(p=.0010), TNF-a (p=.0405) and LT (p=.0286) were increased in SS patients. The differences between early SS versus late SS were significant for IL-6 (p= .0098) and TNF-a (p=.0484).
Figure 1(B): Cytokine assays were performed as outlined in Materials and Methods. There were no statistically significant differences between the different groups.
Figure 1(C): Cytokine assays were performed as described in Materials and Methods. The statistically significant findings were control versus late SS: TNF-a (p=< 0 p=.0021) p=.0091).>
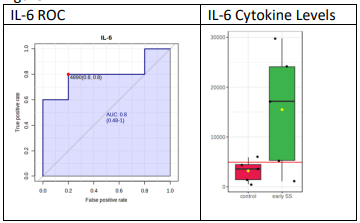
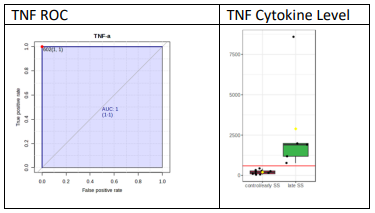
To test whether any of the cytokines can be used as a biomarker for diagnosis, ROC analysis was performed as previously described [13]. Figure 2A demonstrates the ROC analysis and cytokines levels in tears for IL-6 and Figure 2B the ROC analysis and cytokine levels in tears for TNF-a. For this analysis, the higher cytokine values among the two eyes were used. For early SjD vs control, IL-6 had a cut-off value of 4890 fg/ml; but it had one control above the cut-off (5947) and one early SS below the cut-off (1120). So, it can be safely said that anything above 10,000 fg/ml (a conservative estimate) is early SS, but one cannot be sure about what is below 10,000. For late SjD vs control/early SjD, TNF-a gave the best results, with 100% sensitivity and specificity. So, anything above the cut-off value (602 fg/ml) is late SS and anything below is control/early SS. By doing such analysis for all groups (early SjD vs control, late SjD vs early SjD and late SjD vs control/early SjD), the following cytokines in tears may be used as potential biomarkers as follows:
IL-6: 10,000 - 37,500 fg/ml early SjD, ˃ 37,500 fg/ml late SjD
TNF-a: > 602 fg/ml late SjD, < 602>IFN-g: > 200 fg/ml late SjD
TNF-b: > 75 fg/ml late SjD
Figure 2(A): ROC was peformed as described in Materials and Methods. ROC for IL-6 in tears demonstrates that IL-6 levels distinguish between normal controls and pateints with SS. Shown are levels of IL-6 in tears compare normal controls and patients with early SS.
Figure 2(B): ROC was performed as described in Materials and Methods. ROC for TNF-a demonstrates that TNF-a levels in tears distinguish normal controls and early SS patients from long-standing SS patients. Shown are levels of TNF-a in tears compared to normal controls and early SS with late SS.
Discussion
These studies found that cytokines were identified more readily in tears than in saliva or serum. Elevations in IL-6 in tears were characteristic of patients with early SjD while elevations of IL-6, TNF-a and lymphotoxin were noted in patients with long-standing SjD. In serum, TNF-a and IL-2 were elevated only in patients with long-standing SjD. In saliva, there were no statistically significant changes for any of the cytokines tested. Thus, we found that different cytokine profiles characterize SjD in early versus long-standing disease.
The results from this study pointed out several interesting observations. First, SjD is underdiagnosed as many patients who signed up as “normal controls” actually had underlying SjD. It is unclear whether these volunteers participated in the study because they thought they might have SjD, although they did not complain of dry eyes or dry mouth. This is consistent with previous reports that the diagnosis of SjD often occurs only after patients have had problems for 1-2 years and many patients fail to be seen by physicians because they view their early symptoms as minor annoyances rather than as problems requiring attention [1, 5, 16-19]. The second interesting observation from these studies is that many patients who meet clinical criteria for SjD, do not have SSA or SSB antibodies, but do have antibodies to SP1, CA6 and or PSP. This is similar to what has been observed in several studies [12, 20-23]. There was one normal control participant who demonstrated an antibody to CA6, and two to PSP. This might be a false positive or indicative of a predisposition to develop SjD, as has been see in another study of patients expressing SSA and SSB antibodies years before they developed clinically evident SS [15]. The third interesting finding is the difference in the cytokines noted in the tears, saliva and serum of patients. In this study, the tears were the best place to identify abnormal presence of cytokines in patients with both early and late Sjogren’s disease. Finally, of interest is the variability in the cytokines observed in this study compared to other studies.
The role of cytokines has been extensively studied in SjD for many years. For example, interleukin -2 has been shown to function abnormally in SjD and giving IL-2 to an experimental model for SjD to induce Treg cells has been shown to be beneficial [24, 25]. Serum and salivary levels of IL-6 have been shown to be elevated in patients with SjD, but the significance of this finding has not been elucidated [26]. We saw elevated levels of IL-6 in the tears of our patients with SjD, but not in the saliva or serum. Interleukin 17 has been considered a major driver for SjD both in patients and in animal models [27-31]. We did not see elevated levels of IL-17 in the tissues of SjD patients. TNF-a has been shown to be elevated in the saliva of patients with SjD [32], but anti-TNF therapy has failed in previous trials in SjD [33]. In this study, we identified elevated levels of TNF- in the tears and sera of patients with late SjD, but not early SjD. Abnormalities of LT, or TNF-, has been observed in patients with SjD and animal models for SjD [34,35]. Inhibiting LT in an animal model of SjD eliminated features of SjD [35,36], but a soluble LT beta receptor failed in a clinical trial for SjD [37]. In this study we noted elevated LT in the tears of patients with late SjD. Type 1 interferon has been extensively studied as a driver for SjD [4,38-40]. Expression of type 1 interferon has been shown to distinguish different stages of SjD [41]. Treatment of SjD patients with type 1 interferon has been beneficial in some studies [42,43], while current studies evaluating blocking type 1 interferon in SjD patients are having mixed results [44]. In the current studies we did not see elevations in the levels of type 1 interferons in any of the tissues. We also did not see elevations in INF-. Interestingly INF-can play both synergistic and antagonistic roles to type 1 interferons [45,46].
When identifying cytokines in the tissues of patients, it is never clear whether elevated cytokine levels are part of a pathologic process or an attempt of the immune system to correct a pathologic process. Furthermore, the measurement of cytokines in patients with SjD is variable possibly related to differences in the nature of the patients being studied, as well as possibly due to differences in the techniques used to measure cytokine levels. Use of cytokine inhibitors in SjD will require the demonstration of elevations in those cytokines in the individual patients being treated. Furthermore, cytokine abnormalities may differ in different tissues in the same individual. In our studies, more abnormalities were identified in tears than in saliva or sera. This would provide rationale to develop tools to inhibit particular cytokines selectively in particular tissues, such as the eyes.
In terms of utilizing a combination of autoantibody studies and cytokine assays to diagnose SjD, this study suggests that a combination of the two types of studies would be superior to either alone. Autoantibodies SSA, SSB, anti-SP1, anti-CA6 and anti-PSP should be used in combination with levels of IL-6, TNF and LT in tears. However, in order to identify the earliest and hence most treatable SS patients, other types of markers may be necessary as many of the cytokine markers were absent in early SjD patients. Exploring metabolic markers for early disease will likely be a fruitful undertaking [47-51].
Acknowledgements
This work was supported from a grant from Amgen (Award 81087;Study 00001441) along with additional support from Trinity Biotech(Award 94973; Project 1176516).
References
- Vivino, F. B. V. Y. Bunya, G. Massaro-Giordano, C. R. Johr, S. L. Giattino. et.al. (2019). Sjogren's syndrome: An update on disease pathogenesis, clinical manifestations and treatment. Clinical immunology, 203:81-121.
Publisher | Google Scholor - Mavragani, C. P. and H. M. Moutsopoulos. (2020). Sjogren's syndrome: Old and new therapeutic targets. Journal of Autoimmunity, 110.
Publisher | Google Scholor - Maria, N. I. C. G. van Helden-Meeuwsen, E. C. Steenwijk, A. S. Ijpma, Z. Brkic. et.al. (2015). Systemic Interferon Type I and Type II Signatures Present in Distinct Subsets of Primary Sjogren's Syndrome: En Route Towards More Selective Targeting. Arthritis & Rheumatology, 67.
Publisher | Google Scholor - Bave, U. G. Nordmark, T. Lovgren, J. Ronnelid, S. Cajander. et.al. (2005). Activation of the type I interferon system in primary Sjogren's syndrome - A possible etiopathogenic mechanism. Arthritis and rheumatism, 52:1185- 1195.
Publisher | Google Scholor - Akpek, E. K. V. Y. Bunya, and I. J. Saldanha. (2019). Sjogren's Syndrome: More Than Just Dry Eye. Cornea, 38:658-661.
Publisher | Google Scholor - Goules, A. V, and A. G. Tzioufas. (2017). Primary Sjogren's syndrome: clinical phenotypes, outcome and the development of biomarkers. Immunologic Research, 65:331-344.
Publisher | Google Scholor - Nguyen, C. Q. A. Sharma, B. H. Lee, J. X. She, R. A. McIndoe. Et.al. (2009). Differential gene expression in the salivary gland during development and onset of xerostomia in Sjogren's syndrome-like disease of the C57BL/6.NOD-Aec1Aec2 mouse. Arthritis Res Ther, 11:56.
Publisher | Google Scholor - Xuan, J. X. L. Shen, K. Malyavantham, O. Pankewycz, J. L. Ambrus. et.al. (2013). Temporal histological changes in lacrimal and major salivary glands in mouse models of Sjogren's syndrome. Bmc Oral Health, 13.
Publisher | Google Scholor - Agrawal, R. P. K. Balne, A. Veerappan, V. B. Au, B. Lee. Et.al. (2016). A distinct cytokines profile in tear film of dry eye disease (DED) patients with HIV infection. Cytokine, 88:77-84.
Publisher | Google Scholor - Han, Z. A. Christiansen, Meena, and G. Liau. (2021). Relative Quantification of NaV1.1 Protein in Mouse Brains Using a Meso Scale Discovery-Electrochemiluminescence (MSD- ECL) Method. Bio Protoc, 11:3910.
Publisher | Google Scholor - Shen, L. and L. Suresh. (2017). Autoantibodies, detection methods and panels for diagnosis of Sjogren's syndrome. Clinical immunology, 182:24-29.
Publisher | Google Scholor - Zou, K. H, A. J. O'Malley, and L. Mauri. (2007). Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation, 115:654-657.
Publisher | Google Scholor - Shiboski, C. H, S. C. Shiboski, R. Seror, L. A. Criswell. et.al. (2017). 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjogren's Syndrome A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis & Rheumatology, 69:35-45.
Publisher | Google Scholor - Theander, E. R. Jonsson, B. Sjostrom, K. Brokstad, P. Olsson. et.al. (2015). Prediction of Sjogren's Syndrome Years Before Diagnosis and Identification of Patients with Early Onset and Severe Disease Course by Autoantibody Profiling. Arthritis & Rheumatology, 67:2427-2436.
Publisher | Google Scholor - Bunya, V. Y, M. Massaro-Giordano, F. B. Vivino, M. G. Maguire. et.al. (2019). Prevalence of Novel Candidate Sjogren Syndrome Autoantibodies in the Penn Sjogren's International Collaborative Clinical Alliance Cohort. Cornea, 38:1500-1505.
Publisher | Google Scholor - Jonsson. R, K. A. Brokstad, M. V. Jonsson, N. Delaleu, and K. Skarstein. (2018). Current concepts on Sjogren's syndrome - classification criteria and biomarkers. European Journal of Oral Sciences, 126:37-48.
Publisher | Google Scholor - Soliotis, F. C. and H. M. Moutsopoulos. (2004). Sjogren's syndrome. Autoimmunity, 37:305-307.
Publisher | Google Scholor - Vivino, F. B. (2017). Sjogren's syndrome: Clinical aspects. Clinical immunology, 182:48-54.
Publisher | Google Scholor - Martin-Nares, E. and G. Hernandez-Molina. (2019). Novel autoantibodies in Sjogren's syndrome: A comprehensive review. Autoimmunity Reviews, 18:192-198.
Publisher | Google Scholor - Karakus, S. A. N. Baer, and E. K. Akpek. (2019). Clinical Correlations of Novel Autoantibodies in Patients with Dry Eye. Journal of Immunology Research.
Publisher | Google Scholor - Hubschman, S. M. Rojas, M. Kalavar, A. Kloosterboer, A. L. Sabater. et.al. (2020). Association Between Early Sjogren Markers and Symptoms and Signs of Dry Eye. Cornea, 39:311-315.
Publisher | Google Scholor - Applbaum, E. and A. Lichtbroun. (2019). Novel Sjogren's autoantibodies found in fibromyalgia patients with sicca and/or xerostomia. Autoimmunity Reviews, 18:199-202.
Publisher | Google Scholor - Luo, J. B. X. Ming, C. Zhang, X. F. Deng, P. F. Li. et.al. (2018). IL-2 Inhibition of Th17 Generation Rather Than Induction of Treg Cells Is Impaired in Primary Sjogren's Syndrome Patients. Frontiers in immunology 9.
Publisher | Google Scholor - Yao. Y, Y. C. Liang, Y. B. Jin, and J. He. (2018). Low-dose IL-2 administration expands regulatory T cells and protects against experimental Sjogren's syndrome. Clinical and experimental rheumatology, 36:299-299.
Publisher | Google Scholor - Benchabane, S. A. Boudjelida, R. Toumi, H. Belguendouz, P. Youinou. et.al. (2016). A case for IL-6, IL-17A, and nitric oxide in the pathophysiology of Sjogren's syndrome. International Journal of Immunopathology and Pharmacology, 29: 386-397.
Publisher | Google Scholor - Mieliauskaite, D. I. Dumalakiene, R. Rugiene, and Z. Mackiewicz. (2012). Expression of IL- 17, IL-23 and Their Receptors in Minor Salivary Glands of Patients with Primary Sjogren's Syndrome. Clin Dev Immunol.
Publisher | Google Scholor - Verstappen, G. M, O. B. J. Corneth, H. Bootsma, and F. G. M. Kroese. (2018). Th17 cells in primary Sjogren's syndrome: Pathogenicity and plasticity. J Autoimmun, 87:16-25.
Publisher | Google Scholor - Xiao. F, W. H. Du, X. X. Zhu, Y. Tang, L. X. Liu. (2021). IL- 17 drives salivary gland dysfunction via inhibiting TRPC1-mediated calcium movement in Sjogren's syndrome. Clinical & Translational Immunology, 10.
Publisher | Google Scholor - Alunno, A. F. Carubbi, O. Bistoni, S. Caterbi, E. Bartoloni. et.al. (2016). Interleukin (IL)-17-producing pathogenic T lymphocytes co-express CD20 and are depleted by rituximab in primary Sjogren's syndrome: a pilot study. Clinical and Experimental Immunology, 184:284-292.
Publisher | Google Scholor - Nguyen. C. Q, M. H. Hu, Y. Li, C. Stewart, and A. B. Peck. (2008). Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjogren's syndrome: findings in humans and mice. Arthritis and rheumatism, 58:734-743.
Publisher | Google Scholor - Karabulut. G, G. Kitapcioglu, O. Ozcaka, E. Alpoz, A. Nalbantsoy. et.al. (2018). Saliva levels of caspase-1, TNF-alpha, and IFN-gamma in primary Sjogren's syndrome: oral mucosal abnormalities revisited. Turkish Journal of Medical Sciences, 48:554-559.
Publisher | Google Scholor - Nocturne. G, D. Cornec, R. Seror, and X. Mariette. (2016). Use of Biologics in Sjogren's Syndrome. Rheum Dis Clin North Am, 42:407-417.
Publisher | Google Scholor - Hjelmervik, T. O. R, K. Petersen, I. Jonassen, R. Jonsson. (2005). Gene expression profiling of minor salivary glands clearly distinguishes primary Sjogren's syndrome patients from healthy control subjects. Arthritis and rheumatism, 52:1534- 1544.
Publisher | Google Scholor - Shen. L, L. Suresh, J. Wu, J. X. Xuan, H. Li. et.al. (2010). A Role for Lymphotoxin in Primary Sjogren's Disease. Journal of immunology, 185: 6355-6363.
Publisher | Google Scholor - Jacob. A, L. Shen, J. He, K. Nealon, J. Alexander. et.al. (2022). Effect of lymphotoxin blockage on different stages of Sjogren’s syndrome in an animal model. Rheumatology & Autoimmunity, 2:76-81.
Publisher | Google Scholor - St Clair. E. W, A. N. Baer, G. Noaiseh, A. Parke, A. Coca. et.al. (2015). The Clinical Efficacy and Safety of Baminercept, a Lymphotoxin-Beta Receptor Fusion Protein, in Primary Sjogren's Syndrome: Results from a Randomized, Double-Blind, Placebo-Controlled Phase II Trial. Arthritis & Rheumatology, 67.
Publisher | Google Scholor - Bodewes, I. L. A, A. Bjork, M. A. Versnel, and M. Wahren-Herlenius. (2021). Innate immunity and interferons in the pathogenesis of Sjogren's syndrome. Rheumatology, 60: 2561-2573.
Publisher | Google Scholor - Del Papa, N. A. Minniti, M. Lorini, V. Carbonelli, W. Maglione. et.al. (2021). The Role of Interferons in the Pathogenesis of Sjogren's Syndrome and Future Therapeutic Perspectives. Biomolecules, 11.
Publisher | Google Scholor - Marketos, N. I. Cinoku, A. Rapti, and C. P. Mavragani. (2019). Type I interferon signature in Sjogren's syndrome: pathophysiological and clinical implications. Clinical and experimental rheumatology, 37:185-191.
Publisher | Google Scholor - Ambrus J. J, L. Shen, and L. Suresh. (2011). Distinct Roles for Lymphotoxin and Type 1 Interferon in Sjogren's Disease. 11th International Symposium on Sjogren's Syndrome, 21.
Publisher | Google Scholor - Ship J. A, P. C. Fox, J. E. Michalek, M. J. Cummins, and A. B. Richards. (1999). Treatment of primary Sjogren's syndrome with low-dose natural human interferon-alpha administered by the oral mucosal route: a phase II clinical trial. IFN Protocol Study Group. J Interferon Cytokine Res, 19:943-951.
Publisher | Google Scholor - Ferraccioli, G. F. F. Salaffi, S. De Vita, L. Casatta, C. Avellini. (1996). Interferon alpha-2 (IFN alpha 2) increases lacrimal and salivary function in Sjogren's syndrome patients. Preliminary results of an open pilot trial versus OH-chloroquine. Clinical and experimental rheumatology, 14:367-371.
Publisher | Google Scholor - Ritter, J. Y. Chen, A. L. Stefanski, and T. Dorner. (2022). Current and future treatment in primary Sjogren's syndrome - A still challenging development. Joint Bone Spine, 89:105406.
Publisher | Google Scholor - Sen, N. P. Sung, A. Panda, and A. M. Arvin. (2018). Distinctive Roles for Type I and Type II Interferons and Interferon Regulatory Factors in the Host Cell Defense against Varicella- Zoster Virus. Journal of Virology, 92.
Publisher | Google Scholor - Krause, I. G. Valesini, R. Scrivo, and Y. Shoenfeld. (2003). Autoimmune aspects of cytokine and anticytokine therapies. Amer J Med, 115:390-397.
Publisher | Google Scholor - Suresh, L. J. Ambrus, L. Shen, and S. Vishwanath. (2014). Metabolic Disorders Causing Fatigue in Sjogren's Syndrome. Arthritis & Rheumatology, 66:1113-1113.
Publisher | Google Scholor - RamosCasals, M. P. BritoZeron, A. Siso, A. Vargas, E. Ros. et.al. (2007). High prevalence of serum metabolic alterations in primary Sjogren's syndrome: Influence on clinical and immunological expression. The Journal of rheumatology 34: 754-761.
Publisher | Google Scholor - Ambrus J. L, A. Jacob, G. A. Weisman, and J. He. (2018). Metabolic changes in the evolution of Sjogren's syndrome in a mouse model. Clinical and experimental rheumatology, 36:301-301.
Publisher | Google Scholor - Ambrus J. J, P. J. Isackson, M. Moore, J. Butsch, and L. Balos. (2020). Investigating Fatigue and Exercise Inotolerance in a University Immunology Clinic. Archives of Rheumatology and Arthritis Research, 1:1-8.
Publisher | Google Scholor - Bax. K, P. J. Isackson, M. Moore, and J. L. Ambrus. (2020). Carnitine Palmitoyl Transferase Deficiency in a University Immunology Practice. Current Rheumatology Reports,22.
Publisher | Google Scholor