Research Article
Evaluating Autonomic Dysfunction in Alzheimer’s Disease Patients
- Sajal Raj Singh 1
- Nandan Chandregowda 1
- BS KrishnaPrasad 1
- Paalki Sethi 1
- Nalini A 2
- Talkad N Sathyaprabha 1
- Kaviraja Udupa 1*
1Department of Neurophysiology and Neurology, National Institute of Mental Health and Neuro Sciences, Hosur Road, Bangalore India.
*Corresponding Author: Kaviraja Udupa
Citation: Singh S R, Chandregowda N, Prasad BS K, Sethi P, Udupa K, et, al. (2024). Evaluating Autonomic Dysfunction in Alzheimer’s Disease Patients. Journal of Neuroscience and Neurological Research. BioRes Scientia Publishers. 3(2):1-7. DOI: 10.59657/2837-4843.brs.24.020
Copyright: © 2024 Kaviraja Udupa, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: May 21, 2024 | Accepted: June 04, 2024 | Published: June 11, 2024
Abstract
Alzheimer’s disease is an irreversible neurodegenerative disorder with two major protein abnormalities that are amyloid-β (Aβ) deposition and tau accumulation. The vascular hypothesis states that cardiovascular diseases are an important causal or contributing factor in AD, with hypertension regarded as the most powerful vascular risk factor for AD. This study was conducted at the Autonomic Function Lab in the Department of Neurophysiology, National Institute of Mental Health, and Neuroscience (NIMHANS) in Bangalore, India. The AFT results were compared between AD group and healthy volunteers with similar age and sex ratio (n=30).The role of environment and lifestyle are the leading cause of AD along with genetic disposition. The prevalence and progression of the disease affecting the lives of millions of people all over the world have enabled researchers to establish the involvement of the autonomic nervous system in AD.The data collected via HRV analysis and Cardiac Autonomic Function test showed that AD patients had greater sympathetic activity when compared to the normal healthy group which showed signs of symapthovagal balance between sympathetic and parasympathetic systems which concluded that AD is associated with alterations in autonomic nervous system functioning.
Keywords: alzheimer’s disease; cardiovascular dysfunction; autonomic nervous system; automatic function test; heart rate variability
Introduction
Alzheimer’s disease is an irrevocable, intensifying brain disorder that tends to demolish cognitive functions such as learning and memory in the brain and is a gradually progressive neurodegenerative disorder with significant signs of dementia [1,2] Usually, the symptoms of the disease are seen in the late onset of life, nearly 60s. Besides the two major mechanisms (amyloid-β (Aβ) deposition and tau accumulation), others might contribute to sporadic AD, such as those suggested by the vascular hypothesis [3,4]. This states that cardiovascular diseases are an important causal or contributing factor in AD, with hypertension regarded as the most powerful vascular risk factor for AD. A system that could be implicated in this relationship between vascular disease and AD is the cholinergic system [5,6,7]. The cholinergic system is a crucial regulator of cardiovascular and neurocardiac regulation mediated by autonomic functions, and it is prominently affected in AD, beginning in the pre-clinical phases [8,9]. However, early onset of symptoms is also reported in the late 30s in some cases. Another significant aspect that can be associated with the gradual progression of the disease or occurrence of the disease is autonomic dysfunction [10].The transient increase in systemic blood pressure is assessed by the sense of pressure changes in the arterial wall. The information retrieved from the baroreceptor (type of mechanoreceptor) is relayed to the nucleus tractus solitarius and to the vasomotor control centre in the brain [11]. The increase in pressure of the blood causes the arterial wall receptor to sense tension undergoing stretch which causes the transmission of an impulse to increase in the vasomotor region which causes the sympathetic system to decrease its outgoing impulse which ultimately leads to an increase in vagal tone on the SA node. Baroreflex sensitivity is typically measured after a change in blood pressure has occurred. The measurement of sensitivity is found by the change in the R-R Interval measured in the ECG recordings. By assessing the baroreflex sensitivity it can convey the information of how much control the baroreflex has over the heart rate and hence can be used for diagnosis of many cardiovascular ailments [12,13]. The strong association between the progression of age causing diminished cerebral perfusion further leads to a reduction in cerebral blood flow from the heart to the brain, enhancing vascular-related risk factors [14,15]. It has also been indicated by various evidence that chronic brain hypoperfusion is also responsible for the synthesis of defective proteins and defects in protein synthesis which results in the classic pathology of AD, that is, excessive formation of amyloid-β plaques and neurofibrillary tangles which eventually leads to neurodegenerative lesions in AD [16]. One of the important striking manifestations of autonomic system dysfunction in AD is orthostatic hypotension, a condition characterized by a drop in blood pressure when the patient stands up. This phenomenon occurs due to an impaired sympathetic response that results in inadequate blood flow to the brain [17,18]. Orthostatic hypotension poses a significant risk to the safety and well-being of AD patients and requires a thorough understanding and proactive management of this condition. People with AD may develop changes in autonomic cardiac function in addition to orthostatic hypotension. These individuals frequently have abnormal heart rate variability, a reduced heart rate response to exercise, and an elevated heart rate during rest. These abnormalities in autonomic cardiac function put the already precarious health of AD patients at considerably greater risk for cardiovascular events [19]. In addition, Autonomic Dysfunction can cause abnormal thermoregulation, urinary dysfunction, and gastrointestinal dysfunction can all result from autonomic dysfunction in AD.The Cardiovascular Autonomic Function Test (AFT) evaluates and assesses the body’s autonomic functioning using non-invasive methods. The ANS governs many of the physiological processes in our body and has functional aspects in maintaining homeostasis in almost all regions of the body [20,21]. These tests can understand the body’s autonomic system functioning by analysing data revealed from processing certain tests related to cardiovascular functioning. Heart rate feedback to deep breathing and the Valsalva maneuverer helps in assessing cardiovagal functioning. Various tests are assessed under AFT related to various body systems like the cardiovascular, gastrointestinal, sudomotor, and neuroendocrine, etc. An imbalance in the cardiac innervation of the ANS causes instability in normal blood pressure reflexes and heart rate [22]. This has caused many tests to be involved in the assessment of
Methods
Subjects
The study was conducted in the Autonomic Function Lab in the Department of Neurophysiology, National Institute of Mental Health, and Neuroscience (NIMHANS) In Bangalore, India. The patients recruited in the study were diagnosed by a neurologist from the NIMHANS Neurology Department. Previously attained data with recently collected data was organized by the lab technician of the autonomic Function laboratory. For the control group, healthy volunteers were recruited with the same age and sex parameters. The patients and the control subjects were comparable in their socio-demographic parameters. Patients diagnosed with Alzheimer’s disease by Neurologist (AN) included in this study. On the other hand, patients that have artefacts in their ECG reports, or patients unable to perform these tests excluded. In this retrospective study protocol, patients AFT Laboratory database was screened and clinical details including demographic data relating to age and gender were also collected. The AFT results of 15 AD patients were collected in one group which was put under-treated group. 15 healthy volunteers’ data were collected from the healthy subject’s database as control group. Both the data groups were compared using unpaired student t-test. Parameters (Heart rate variability measures, Cardiac autonomic function test, Blood pressure assessment).All the tests were conducted using LabChart pro version 8.1 and the analysis was done for a particular mid-range duration of 15-20 minutes for HRV data.
Autonomic Function test
All the subjects for the tests of the autonomic relations taken for the study were conducted in the Autonomic lab, Department of Neurophysiology, NIMHANS under the standardized conditions. The test was conducted during the lab official hours from 9:00 am to 4:30 pm. Before the test was conducted the subjects were advised in advance about their diet condition, like having light breakfast or lunch and no intake of any caffeinated products, and divesting of bladder and bowel before the test is to be conducted. The subjects were informed about what the test is and why it is necessary to perform, and all the queries of the subjects were answered [27].
Heart Rate Variability (HRV)
Heart rate variability is the variation in the fluctuation between each heartbeat and usually recorded by measuring the duration of time between each heartbeat. This phenomenon is in the natural heart process. As discussed earlier how the heart rate is under the influence of the ANS any changes in the innervation of the parasympathetic and sympathetic nervous system can cause changes in the activity of the cardiovascular function including changes in the HRV. Hence HRV assessment can be used as an important tool for assessing the alteration in cardiac autonomic function [28]. First, the data for HRV analysis was done by selecting an artifact-free segment of 5 minutes’ duration after the first 3 minutes and the point care graph plot was selected within the ECG frame and then the analysis was done. The analysed data was then added to the data pad and the data pad was viewed. This step was repeated 3 times for the successive three segments and the data was analysed and interpreted in the report.
Deep Breathing Test (DBT)
Based on the phenomenon of respiratory arrhythmia which is to be performed in a duration of a minute where each minute should only constitute 6 breath cycle. Where the subjects were asked to slowly inhale and slowly exhale, each at a duration of 5 seconds. The results of these were found to be affected by many factors age, disease, and cardiovascular health. The ECG segment of the deep breathing test was selected where 6 peaks of inspiration and expiration could be seen. Deep breathing difference (DBD) was calculated from the mean of the differences between maximum heart rate during inspiration and minimum heart rate for 6 such inspiration-expiration cycles.
Valsalva Ratio (VR)
This test was calculated based on the activity of a technique called the Valsalva maneuver which helps in assessing the function of the baroreceptors. This technique is described as a voluntary forced expiration. Where the subject was instructed to forcefully blow hard into a tube with maintaining strong pressure. This caused a transient increase in blood pressure categorized and demonstrated in (phase I) which caused slight bradycardia due to the activation of the baroreceptors followed by a decrease in blood pressure with compensatory tachycardia (phase II) and the stopped expiration is regarded (phase III).
Isometric Hand Grip Test (IHG)
The subjects were given an IHG device that the individual holds firmly to apply pressure while keeping his/her hand in and upright position straight for a duration of 2 minutes. A basal BP was recorded before the test is initiated and after 2 minutes of IHG another BP reading was done, and the test was recorded. There is an increase in diastolic pressure usually happens when performing a handgrip test for a duration of 2-3 minutes. The change in diastolic pressure occurs due to heart rate acceleration.
Orthostatic test (OST)
During this test, the subjects were asked to go from a supine position to an active standing position within 3 seconds. So as soon as the subject stands up a high amount of blood rushes to the blood vessels toward the lower body which causes the cardiac stroke volume to decrease in return. During this process, it is accompanied by two events which is a sudden fall in systolic and diastolic blood pressure and an abrupt increase in heart rate which is carried on by a stabilization phase. These phases make up a peak with a downward drop and a stabilization phase to record the heart rate at the peak and select the next heart rate value at 15 seconds from the initial point of value. The ratio of the two values were then calculated.
Statistical Analysis
The data was analysed by using Graph Pad Prism 9 Software. The autonomic function test results between AD patients (treated) and healthy volunteers (controlled) were compared by performing non-parametric unpaired t-test for each parameter comparing each of the variables between the groups. Correlation between the progression of AD and corresponding autonomic parameters were studied and analysed using Pearson's correlation coefficient Alpha (∝), set at 5% (p less than 0.05)
Results
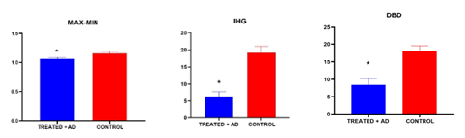
This is a case-control study within two groups, one with AD patients (n=15) that were compared with healthy individuals (n=15) with similar age and sex parameters. Comparison of demographic profiles between AD (n=15) and Controlled groups (n=15) for age 65.26 ± 9.38, 64.8 ± 9.00, and gender 9:06 for both the groups.Non-parametric unpaired t-test was performed for each parameter comparing each of the variables between the groups, according to statistical analysis there is no significance when compared for the time domain of HRV measure for SDNN (Standard deviation of N-N intervals), RMSSD (Square root of the mean of the sum of squares of differences between NN intervals) and resting heart rate. Although, it was found that the AD patient group had an overall slightly higher resting heart rate above the mean value when compared to the control group of individuals. When both the groups were compared for the frequency domain of the HRV measure, it was found that there is no statistical significance for total power, low-frequency power, high-frequency power (normalized units), and low-frequency/high-frequency ratio. However, in AD patients it was found that they had a sympathetic dominance side revealing a higher total power and an alteration in normal sympathetic and parasympathetic tone from low and high-frequency measures. In the conventional autonomic function test, according to statistical analysis applied in both the groups for comparison it was found that Deep breathing difference (DBD) was significant indicating cardiac autonomic abnormalities in AD patients. IHG data revealed statistically significant differences when the values were compared between the two groups revealing the alteration of the normal cardiac autonomic dysfunction. In the maximum-minimum ratio of the orthostatic test, statistical significance was found between the correlation of AD patients with cardiac autonomic abnormalities. While in the orthostatic test and Valsalva ratio (VR) there was no statistical significance.
Table 1: Shows the HRV Parameters and Cardiac Autonomic function test comparison between the given groups, AD patients and normal healthy individuals. (Statistical significance has been shown via * that denotes significance level <0>
| PARAMETERS | AD (n=15) | Controls (n=15) | T-test | P value |
| SDNN | 29.02 ± 18.53 | 26.96 ± 15.05 | 0.7417 | 0.4473 |
| RMSSD (27 ± 12) | 16.30 ± 12.27 | 20.48 ± 11.78 | 0.3486 | 0.8648 |
| TOTAL POWER | 1175.01 ±1323.87 | 1060.78±1556.92 | 0.8301 | 0.5520 |
| LOW-FREQUENCY POWER (1170 ± 416) | 172.57 ± 214.27 | 224.55 ± 321.38 | 0.6063 | 0.6741 |
| HIGH-FREQUENCY POWER (975 ± 203) | 131.268 ±222.295 | 172.30 ± 173.31 | 0.5774 | 0.7579 |
| LOW FREQUENCY (54± 4) | 57.97 ± 19.14 | 48.37 ± 17.07 | 0.1583 | 0.1414 |
| HIGH FREQUENCY (29 ± 3) | 35.721 ± 17.741 | 45.14 ±16.31 | 0.1412 | 0.3630 |
| LF/HF RATIO (0.5 -1.5) | 2.952 ± 4.376 | 1.24 ± 0.74 | 0.1469 | <0> |
| DBD (>15) | 8.508 ± 6.112 | 18.16 ± 5.45 | 0.0003* | 0.6780 |
| VR (1.21) | 1.194 ± 0.174 | 1.3 ± 0.8 | 0.1173 | <0> |
| OST (max &min) (>1.04) | 1.072 ± 0.08 | 1.15 ± 0.108 | 0.0214* | 0.3031 |
| IHG (>15) | 6.2 ± 4.61 | 19.13 ± 7.21 | 0.000015* | 0.1799 |
| OST <10> | 1.428 ± 17.42 | 12.46 ± 14.81 | 0.0786 | 0.5551 |
SDNN: standard deviation of all NN intervals, RMSSD: square root of the mean of the sum of squares of differences between adjacent NN intervals, LF: Low frequency power, HF: High frequency power, DBD: Deep Breathing difference, VR: Valsalva ratio, OST (max & min): Orthostatic test maximum and minimum ratio, IHG: isometric hand grip test, OST: Orthostatic Test.
Figure 1-3: Graphical representation of DBD, MAX-MIN, IHG parameters comparison between the two groups treated vs controlled.There was statistical significance seen between the given groups for DBD: Deep Breathing Difference, MAX-MIN: Orthostatic maximum and minimum ratio, IHG: Isometric Handgrip Test.
Discussion
Our study was performed between the two groups of 15 subjects each with a gender and age match group revealed that there was involvement of dysautonomia in patients with AD. The data collected showed that AD patients had greater sympathetic activity when compared to the normal healthy group which showed signs of sympathovagal balance between sympathetic and parasympathetic systems [29], suggesting parasympathetic suppression and sympathetic over expression. However, HRV analysis did not reveal any significant differences between the two groups of data. Although, the HRV analysis has been seen as an assessment tool for diagnosing many cardiac related dysfunctions which has revealed dysfunction in the autonomic nervous system.The total power of the HRV analysis had a greater average when compared to the control group, since total power revealed the sum of all the frequency-related factors and AD patients have a sympathetic dominance side revealing a higher total power. The LF value indicates sympathetic and parasympathetic tone having value lower than normal data value was indicative of alteration in normal sympathetic and parasympathetic tone which is justified by a mean towards lower side in AD. The parasympathetic tone was altered from normal neurophysiology of the system in AD patients suggested by lower HF value of mean. The LF/HF value was seen to be towards the greater side when compared to the normal values which was indicative of average sympathetic dominance in AD group of patients [30]. Since Alzheimer’s Disease has been discussed as the disease of the elderly and it’s a well-known fact that cardiovascular system efficiency decreases with the progression of age. The cardiovascular tone weakens and most of the normal outcome is not maintained. In the present study, AD patients are elderly people aged 65.26 ± 9.38 and the question that arises is that the values that are obtained are confirmatory of age-related abnormalities or due to the condition of Alzheimer's Disease related dysfunction. Well, the dysfunction by HRV analysis categorized present are not significant hence alone HRV alone cannot be used to ascertain any diagnosis of AD. However, that data does reveal some infractions from normal values hence are indicative of both age-related Alzheimer's disease as AD has been termed as the “Disease of the Elderly” [31]. The clinical understanding following HRV testing is a broad term. There are various factors to testing that may cause interference with the test results. The memory which has been seen to be positively linked to parasympathetic power and negatively to sympathetic power was hence seen to be true because of the greater sympathetic output in AD patients [32].The cardiac autonomic function test was very indicative of the significant changes that were observed between the comparison of given groups. The DBD test indicated a statistically significant value change which further suggested cardiac autonomic dysfunction. IHG data revealed statistically significant differences when the values were compared between the two groups revealing the alteration of the normal cardiac autonomic dysfunction. The MAX-MIN ratio derived from orthostatic test was seen to be statistically significant and that was indicative of the cardiac autonomic dysfunction [33].The cardiac autonomic test revealed many significant value differences which showed the alteration in the autonomic nervous system. According to Ewing’s classification of autonomic failure all the tests performed for AD patient group showed definitive to severe abnormality in the cardiac function assessment. The test revealed those changes because the test involved people to undergo various form of cardio-active form of activity like deep breathing for heart rate changes and isometric hand grip test that involved the muscle activity response for cardiovascular assessment showed alteration different than the normal values [34]. However, these tests required cooperation of patients but due to memory loss cases in the demented patients it has provided negative impact. Abnormalities in the homeostasis of AD patients were seen which further adding up to dysfunctioning in the sympathetic and parasympathetic functions [35]. Hence concluding the remark of cardiac autonomic alteration in AD patients’ group during the study. Hence according to our results, it can be said that AD is a common disorder that affect elderly who have been associated with other comorbidities due to the progression of age factors which causes cardiovascular function decline which interferes with autonomic function. Limitation of the study which could have benefited the study were lower sample size, the presence of uncontrollable variables such as age, and other undiagnosed abnormalities in AD patients, and assessment at different stages of AD was not assessed.
Discussion
Our study was performed between the two groups of 15 subjects each with a gender and age match group revealed that there was involvement of dysautonomia in patients with AD. The data collected showed that AD patients had greater sympathetic activity when compared to the normal healthy group which showed signs of sympathovagal balance between sympathetic and parasympathetic systems [29], suggesting parasympathetic suppression and sympathetic over expression. However, HRV analysis did not reveal any significant differences between the two groups of data. Although, the HRV analysis has been seen as an assessment tool for diagnosing many cardiac related dysfunctions which has revealed dysfunction in the autonomic nervous system.The total power of the HRV analysis had a greater average when compared to the control group, since total power revealed the sum of all the frequency-related factors and AD patients have a sympathetic dominance side revealing a higher total power. The LF value indicates sympathetic and parasympathetic tone having value lower than normal data value was indicative of alteration in normal sympathetic and parasympathetic tone which is justified by a mean towards lower side in AD. The parasympathetic tone was altered from normal neurophysiology of the system in AD patients suggested by lower HF value of mean. The LF/HF value was seen to be towards the greater side when compared to the normal values which was indicative of average sympathetic dominance in AD group of patients [30]. Since Alzheimer’s Disease has been discussed as the disease of the elderly and it’s a well-known fact that cardiovascular system efficiency decreases with the progression of age. The cardiovascular tone weakens and most of the normal outcome is not maintained. In the present study, AD patients are elderly people aged 65.26 ± 9.38 and the question that arises is that the values that are obtained are confirmatory of age-related abnormalities or due to the condition of Alzheimer's Disease related dysfunction. Well, the dysfunction by HRV analysis categorized present are not significant hence alone HRV alone cannot be used to ascertain any diagnosis of AD. However, that data does reveal some infractions from normal values hence are indicative of both age-related Alzheimer's disease as AD has been termed as the “Disease of the Elderly” [31]. The clinical understanding following HRV testing is a broad term. There are various factors to testing that may cause interference with the test results. The memory which has been seen to be positively linked to parasympathetic power and negatively to sympathetic power was hence seen to be true because of the greater sympathetic output in AD patients [32].The cardiac autonomic function test was very indicative of the significant changes that were observed between the comparison of given groups. The DBD test indicated a statistically significant value change which further suggested cardiac autonomic dysfunction. IHG data revealed statistically significant differences when the values were compared between the two groups revealing the alteration of the normal cardiac autonomic dysfunction. The MAX-MIN ratio derived from orthostatic test was seen to be statistically significant and that was indicative of the cardiac autonomic dysfunction [33].The cardiac autonomic test revealed many significant value differences which showed the alteration in the autonomic nervous system. According to Ewing’s classification of autonomic failure all the tests performed for AD patient group showed definitive to severe abnormality in the cardiac function assessment. The test revealed those changes because the test involved people to undergo various form of cardio-active form of activity like deep breathing for heart rate changes and isometric hand grip test that involved the muscle activity response for cardiovascular assessment showed alteration different than the normal values [34]. However, these tests required cooperation of patients but due to memory loss cases in the demented patients it has provided negative impact. Abnormalities in the homeostasis of AD patients were seen which further adding up to dysfunctioning in the sympathetic and parasympathetic functions [35]. Hence concluding the remark of cardiac autonomic alteration in AD patients’ group during the study. Hence according to our results, it can be said that AD is a common disorder that affect elderly who have been associated with other comorbidities due to the progression of age factors which causes cardiovascular function decline which interferes with autonomic function. Limitation of the study which could have benefited the study were lower sample size, the presence of uncontrollable variables such as age, and other undiagnosed abnormalities in AD patients, and assessment at different stages of AD was not assessed.
Conclusion
The role of environment and lifestyle are the leading cause of AD along with genetic deposition. The prevalence and progression of disease affecting the lives of millions of people all over the world have enabled researchers to link the involvement and correlation of the autonomic nervous system. The data collected showed that AD patients had greater sympathetic activity when compared to the normal healthy group which showed signs of sympathovagal balance between sympathetic and parasympathetic systems. These results were nearly identical to the other reviewed findings upon literature review. The HRV report also established the influence of sympathetic dominance in AD patients and suppression of the parasympathetic system. Which showed the shift of the sympathovagal balance towards the output of sympathetic innervation. Which may cause an increased risk of cardio-autonomic dysfunction later in life. The cardiac autonomic testing revealed significant changes in the AD patients showing clearly cardio-autonomic dysfunction in relation to Alzheimer’s Disease.
References
- Carly Oboudiyat, MD Hilary Glazer, MD Alon Seifan, MD, MS Christine Greer, et al. (2013). Alzheimer’s Disease. Semin Neurol, 33(4):313-329.
Publisher | Google Scholor - Idiaquez J, Roman GC. (2011). Autonomic dysfunction in neurodegenerative dementias. J Neurol Sci, 15:305(1-2):22-27.
Publisher | Google Scholor - Omar Serý 1, Jana Povová, Ivan Míšek, Lukáš Pešák, Vladimir Janout. (2013). Molecular mechanisms of neuropathological changes in Alzheimer's disease: a review. Folia Neuropathol, 51(1):1-9.
Publisher | Google Scholor - Beach PA, Huck JT, Miranda MM, Bozoki AC. (2015). Autonomic, Behavioral, and Subjective Pain Responses in Alzheimer's Disease. Pain Med, 16(10):1930-1942.
Publisher | Google Scholor - Allan LM, Ballard CG, Allen J, Murray A, Davidson AW, McKeith IG, Kenny RA. (2007). Autonomic dysfunction in dementia. J Neurol Neurosurg Psychiatry, 78(7):671-677.
Publisher | Google Scholor - David M, Malhotra PA. (2022). New approaches for the quantification and targeting of noradrenergic dysfunction in Alzheimer's disease. Ann Clin Transl Neurol, 9(4):582-596.
Publisher | Google Scholor - Allan LM, Ballard CG, Allen J, Murray A, Davidson AW, McKeith IG, et al. (2007). Autonomic dysfunction in dementia. J Neurol Neurosurg Psychiatry, 78(7):671-677.
Publisher | Google Scholor - H Hampel 1, M-M Mesulam, A C Cuello, A S Khachaturian, A Vergallo, et al. (2019). Revisiting the Cholinergic Hypothesis in Alzheimer's Disease: Emerging Evidence from Translational and Clinical Research. J Prev Alzheimers, 6(1):2-15.
Publisher | Google Scholor - Santos CY, Machan JT, Wu WC, Snyder PJ. (2017). Autonomic Cardiac Function in Preclinical Alzheimer's Disease. J Alzheimer’s, 59(3):1057-1065.
Publisher | Google Scholor - Algotsson A, Viitanen M, Winblad B, Solders G. (1995). Autonomic dysfunction in Alzheimer's disease. Acta Neurol Scand, 91(1):14-8.
Publisher | Google Scholor - Andresen, M.C. (2004). Cardiovascular Integration in the Nucleus of the Solitary Tract. In: Dun, N.J., Machado, B.H., Pilowsky, P.M. (eds) Neural Mechanisms of Cardiovascular Regulation. Springer.
Publisher | Google Scholor - Szili-Török T, Kálmán J, Paprika D, Dibó G, Rózsa Z, Rudas L. (2001). Depressed baroreflex sensitivity in patients with Alzheimer's and Parkinson's disease. Neurobiol Aging, 22(3):435-438.
Publisher | Google Scholor - Ehlen JC, Forman CM, Ostrowski D, Ostrowski TD. (2022). Autonomic Dysfunction Impairs Baroreflex Function in an Alzheimer's Disease Animal Model. J Alzheimers, 90(4):1449- 1464.
Publisher | Google Scholor - Braz ID, Fisher JP. (2016). The impact of age on cerebral perfusion, oxygenation and metabolism during exercise in humans. J Physiol, 15;594(16):4471-83.
Publisher | Google Scholor - Lucy C. Beishon, Patrick Hosford, Dewaker Gurung, Patrice Brassard, Jatinder S. Minhas, Thompson G. Robinson, Victoria Haunton, Ronney B. (2022). Panerai, The role of the autonomic nervous system in cerebral blood flow regulation in dementia: A review, Autonomic Neuroscience, 240:102985.
Publisher | Google Scholor - Jack C. de la Torre. How do heart disease and stroke become risk factors for Alzheimer's disease? Neurological Research.
Publisher | Google Scholor - Isik, A.T., Erken, N., Yavuz, I. et al. Orthostatic hypotension in patients with Alzheimer’s disease: a meta-analysis of prospective studies. Neurol Sci 43:999-1006 (2022).
Publisher | Google Scholor - Tulbă D, Cozma L, Popescu BO, Davidescu EI. (2020). Dysautonomia in Alzheimer's Disease. Medicina (Kaunas), 56(7):337.
Publisher | Google Scholor - Sanna G, Nusdeo G, Piras M, et al. (2019). Cardiac Abnormalities in Alzheimer Disease. J Am Coll Cardiol HF, 7 (2):121-128.
Publisher | Google Scholor - Wehrwein EA, Orer HS, Barman SM. (2016). Overview of the Anatomy, Physiology, and Pharmacology of the Autonomic Nervous System. Compr Physiol, 6(3):1239-1278.
Publisher | Google Scholor - Franceschi M, Ferini-Strambi L, Minicucci F, Sferrazza-Papa A, Smirne S. (1986). Signs of cardiac autonomic dysfunction during sleep in patients with Alzheimer's disease. Gerontology, 32(6):327-334.
Publisher | Google Scholor - Jensen-Dahm C, Waldemar G, Staehelin Jensen T, Malmqvist L, Moeller MM, Andersen BB, Høgh P, Ballegaard M. Autonomic Dysfunction in Patients with Mild to Moderate Alzheimer's Disease. J Alzheimers Dis. 2015;47(3):681-689.
Publisher | Google Scholor - Duong HTH, Tadesse GA, Nhat PTH, Hao NV, Prince J, Duong TD, Kien TT, Nhat LTH, Tan LV, Pugh C, Loan HT, Chau NVV, Minh Yen L, Zhu T, Clifton D, Thwaites L. (2020). Heart Rate Variability as an Indicator of Autonomic Nervous System Disturbance in Tetanus. Am J Trop Med Hyg. 102(2):403-407.
Publisher | Google Scholor - Nonogaki Z, Umegaki H, Makino T, Suzuki Y, Kuzuya M. (2017). Relationship between cardiac autonomic function and cognitive function in Alzheimer's disease. Geriatr Gerontol Int, (1):92-98.
Publisher | Google Scholor - Dias FL, Silva RM, Moraes EN, Caramelli P. Clinical and autonomic profile of patients with Alzheimer's disease and mixed dementia patients. (2013). Rev Assoc Med Bras, 59(5):435--441.
Publisher | Google Scholor - Idiaquez J, Roman GC. (2011). Autonomic dysfunction in neurodegenerative dementias. J Neurol Sci, 305(1-2):22-27.
Publisher | Google Scholor - Udupa K, Sathyaprabha TN, Thirthalli J, Kishore KR, Lavekar GS, et al. (2007). Alteration of cardiac autonomic functions in patients with major depression: a study using heart rate variability measures. J Affect Disord, 100: 137-141.
Publisher | Google Scholor - Tiwari R, Kumar R, Malik S, Raj T, Kumar P. (2021). Analysis of Heart Rate Variability, and Implication of Different Factors on Heart Rate Variability. Curr Cardiol Rev, 17(5): e160721189770.
Publisher | Google Scholor - Rangon CM, Krantic S, Moyse E, Fougère B. (2020). The Vagal Autonomic Pathway of COVID-19 at the Crossroad of Alzheimer's Disease and Aging: A Review of Knowledge. J Alzheimers Dis Rep, 4(1):537-551.
Publisher | Google Scholor - Collins O, Dillon S, Finucane C, Lawlor B, Kenny RA. (2012). Parasympathetic autonomic dysfunction is common in mild cognitive impairment. Neurobiol Aging, 33(10):2324-2333.
Publisher | Google Scholor - Royall DR, Gao JH, Kellogg DL Jr. (2006). Insular Alzheimer's disease pathology as a cause of
Publisher | Google Scholor - Femminella, Grazia Daniela et al. (2014). ‘Autonomic Dysfunction in Alzheimer’s Disease: Tools for Assessment and Review of the Literature’. 369- 377.
Publisher | Google Scholor - Toledo MA, Junqueira LF Jr. (2010). Cardiac autonomic modulation and cognitive status in Alzheimer's disease. Clin Auton Res, 20(1):11-17.
Publisher | Google Scholor - Santos CY, Machan JT, Wu WC, Snyder PJ. (2017). Autonomic Cardiac Function in Preclinical Alzheimer's Disease. J Alzheimers Dis, 59(3):1057-1065.
Publisher | Google Scholor - Juan Idiaquez, Gustavo C. Roman. (2011). Autonomic dysfunction in neurodegenerative dementias. Journal of the Neurological Sciences, 305:22-27.
Publisher | Google Scholor