Research Article
Escherichia coli Isolation, Prevalence, and Multi Drug Resistance from a Poultry Farm in Sendafa Town, Central Ethiopia
- Abdi Ahmed Umer *
- Ebisa Mezgebu Hambisa
Animal Health Institute (AHI), Microbiology Research Laboratory, Sebeta, Ethiopia.
*Corresponding Author: Abdi Ahmed Umer, Animal Health Institute (AHI), Microbiology Research Laboratory, Sebeta, Ethiopia.
Citation: Abdi A. Umer, Ebisa M. Hambisa (2023). Escherichia coli Isolation, Prevalence, and Multi Drug Resistance from a Poultry Farm in Sendafa Town, Central Ethiopia. Journal of BioMed Research and Reports, BRS Publishers. 2(6):1-8. DOI: 10.59657/2837-4681.brs.23.038
Copyright: © 2023 Abdi Ahmed Umer, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: September 07, 2023 | Accepted: September 21, 2023 | Published: September 28, 2023
Abstract
Background: Escherichia coli is bacteria that exist as commensal in the intestine of animals and humans, but pathogenic strains cause disease in chickens. The growth of antimicrobial resistance in E. coli is one of major concern worldwide.
Objective: This study aimed to assess the prevalence, and Multi drug resistance profile and determine the potential risk factor of E. coli isolates from chickens and chicken’s environment in Sendafa town, central Ethiopia.
Methods: A cross-sectional study was carried out from January to August 2023 in Sendafa town. Purposive sampling techniques were used based on the presence of clinical symptoms suggestive of E. coli in chicken farms. Different types of samples were collected including 207 cloacae swabs, 8 feed, and 8 water from different farms. E. coli isolation and identification were done using bacterial culture, and biochemical, and confirmed using the Matrix Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI TOF MS). Antimicrobial susceptibility testing (AST) was done using the Kirby Bauer disc diffusion method. Data were entered in Microsoft Excel and analyzed with descriptive statistics using SPSS version 20.
Result: Out of a total of 223 samples of cloacae swab, feed, and water 71 (31.83 %) were found to be E. coli suspected by biochemical and finally confirmed with Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI TOF MS). All confirmed isolates were subjected to 12 antimicrobial agents to check their susceptibility. From 71 tested Escherichia coli isolates we found resistant patterns of E. coli to 62(87.3%) Amoxicillin, 44(62%) Tetracycline, and 39(54.9%) Streptomycin respectively. Multi-drug resistance was observed in this study for two or more than resistant detected. Some isolate was sensitive to 60(84.5%) Trimethoprim Sulphamethoxazole, 57(80.3%) Norfloxacin, 56(78.9 %) Ceftriaxone, and 53(74.6%) Meropenem respectively. Intermediate antibiotics were observed on 32(45.1 %) Sulfonamides, and 23(32.4%) Gentamicin for Escherichia coli isolate. Risk factors were analyzed with descriptive statistics. In the study, the difference in E. coli prevalence by age and among sample types was not statistically significant (P>0.05). Between kebele and farm, there was a statistically significant difference in E. coli prevalence (P <0.05).
Conclusion: In this study, a high incidence of E. coli and antibiotic resistance to commonly used antibiotics including Amoxicillin, Tetracycline, and Streptomycin in poultry were found. This implies that there is the existence of practices that accelerate antimicrobial resistance in the sampled chickens therefore, appropriate use of antimicrobial agents, good biosecurity measures, and hygiene practices in chicken farms are important.
Keywords: antibiotic resistance; occurrence; escherichia coli; ethiopia; poultry; sendafa
Introduction
Escherichia coli are facultative, anaerobic Gram-negative rods bacteria and one of the typical microbial species found in the gastrointestinal tracts of animals, humans, and poultry [1]. About 10 to 15% of intestinal coliforms are opportunistic and pathogenic, which cause a variety of lesions in immune compromised hosts as well as in poultry [2]. Even though the majority of E. coli isolates are nonpathogenic, and also, cause infections like swollen head syndrome, yolk sac infection and colibacillosis are among the illnesses that are frequently severe and occasionally fatal [3]. One of the main causes of morbidity and mortality in poultry, colibacillosis also leads in a 20% reduction in egg production and hatching rates [4].
Antibiotics are frequently used to control infectious diseases or as growth stimulants in the poultry. Antibiotics can lead to the emergence and dissemination of resistant E. coli which can then be passed into people via direct or indirect contact with infected animals [5]. These resistant microbes may function as a potential source in the transportation of antimicrobial resistance to human pathogens [6]. The overuse of antibiotics in veterinary care has increased the number of bacterial strains that are resistant to treatment. Antibiotics are commonly used to treat bacterial illnesses in both humans and animals. Antibiotics are antimicrobial substances with the ability to either kill or inhibit the growth of germs. There are between 100 and 200 thousand tons of antibiotics produced annually on a global scale [7]. Animal and human isolates around the world frequently contain Multi drug-resistant (MDR) E. coli strains, and the intestine is probably an important repository of resistance genes [8]. In rare cases, drug-resistant E. coli of animal origin may also briefly colonize the human intestine. The management of intra and extra-intestinal infections caused by E. coli, which are a major cause of illness, death, and increased healthcare costs is complicated by acquired multidrug resistance to antimicrobial agents [9].
Therefore, the goal of the current study was to isolate E. coli from poultry and poultry farm environment in Sendafa town in order to determine their prevalence, antimicrobials resistance profile and potential risk factor of E. coli isolates.
Materials & Methods
Study area
A study was carried out in Sendafa Town (Figure 1), central Ethiopia, from January to August 2023 to isolate E. coli from poultry and environment sampling and evaluate their antimicrobial resistance. Sendafa is found in Shagger City, Oromia Regional Government 39 kilometers north of Addis Ababa Ethiopia's capital. It is bordered to the north by Aleltu, to the south and east by Berek, and the west by the town administrations of Laga Dadi and Laga Tafo. The town is located at an elevation of 2514 meters above sea level, between 9 06'14" and 9 10'30" North latitudes and 38 57'60" and 39 04'53" East longitudes. The region experiences 1200 mm of annual rainfall in two distinct seasons (June to August and January to April), and the average temperature is between 15 and 24 0C [10]. The primary farming method in the region was mixed farming. The most prevalent form of animal husbandry in the region was traditional and industrial poultry farming.
Figure 1: Map of study area
Source: Ethiopia district and region shape file data ESRI Shape file
Study design and Population
A cross-sectional study was carried out in Sendafa town, Shagger city, central Ethiopia, from January to August 2023 to evaluate the prevalence, of the pattern of antimicrobial resistance and identify potential risk factors for E. coli isolates from chicken and the poultry environment. The study was conducted on 223 total samples 207 Cloacae swabs of chicken, 8 feed, and 8 water samples. Eleven poultry farms in four kebele in Sendafa Town were included in the study. Purposive sampling techniques were used based on the presence of clinical diarrheal symptoms suggestive of E. coli in chicken farms. The name of the farm, the date of sampling, the type of sample, the age, sex, and an identification number for each sample were all taken down on the sample collection format along with clinical information about chickens.
Samples collection and Transportation
Cloacae swab: All Cloacae swab samples were collected using sterile swabs which were moistened with sterile buffered peptone water (Oxoid, UK), and the chicken's cloacae swab placed in sterile vial tubes containing 9 mL buffered peptone water which is used to avoid drying out of the swabs for further analysis. Poultry feed samples of 10gm were directly collected from feeding traps of different farms. A total of 8 feed samples were aseptically collected and immediately transferred into a sterile Plastic bag. Water samples were taken from the drinking water of chickens, and 10 ml were transferred to sterile test tubes with screw caps. After collection, all of the samples were transported to the Animal Health Institute (AHI), Sebeta, for bacteriological analysis while being kept in ice boxes.
Isolation and Biochemical methods
Isolation of E. coli all collected samples was enriched in Brain heart infusion broth (BHIB) (CRITERION, USA), and incubated for 24 hours at 37 °C. Following that a loop full of sample broth cultures was streaked onto EMB agar (HiMEDIA, India), and the colony of E. coli was examined for a grayish metallic sheen. To conduct biochemical testing, typical colonies were then transferred to Brain heart infusion agar (BHIA). Suspected E. coli pure colonies were used for biochemical that used to test indole, methyl red, Voges Proskauer and citrate. Then Matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI-TOF MS), was used for further confirmation. After confirmation, the isolates were subjected to antimicrobial susceptibility testing. These isolates were then preserved and kept at 20°C for storage[11].
Maldi TOF MS Method
Young, pure single colonies were sub cultured on BHI agar (CRITERION, USA) and incubated at 37°C for 24 hours. For direct sample preparation method, a single bacterial colony placed directly on MSP 96 target plate, at the end of the samples add controls of 1µl Bacterial Test Standard (BTS) (Bruker Daltonik) then dried at room temperature. Following drying add 1µl of HCCA matrix solution (cyano-4-hydroxycinnamic acid, Bruker Daltonik GmbH) on the dried samples and BTS. Then read target plate by MALDI TOF MS (Bruker Daltonik, Germany) and standard calibration spectra were calibrated before reading samples. The E. coli organism was then interpreted with a score value and National Center for Biotechnology Information (NCBI) [12].
Antimicrobial sensitivity testing
E. coli isolates were tested for antimicrobial susceptibility by [13] using the Kirby-Bauer disk diffusion method, and the bacterial isolates were tested for antibiotic susceptibility. A densitometer was used to measure the turbidity, which was then adjusted to 0.5 standards McFarland. Three to five isolated colonies were then transferred to 5 ml of 0.85% sterile saline water. Following the measurement of turbidity, bacteria were evenly swabbed onto the surface of a Mueller-Hinton agar plate (CRITERION, USA) by rotating the sterile cotton swab by 60°. The plates were subsequently left for three to five minutes and then 12 antimicrobial discs (Oxoid, England) including Streptomycin (S;10 μg), Ampicillin (AMP;10 μg), and Gentamicin (CN;10μg) Tetracycline (TE; 30 μg), Norfloxacin (NOR; 10 μg), Amoxicillin clavulanate(AMC; 30 μg), Ceftriaxone (CRO; 300 μg), Meropenem (MEM; 10μg), Sulfonamides (S3; 300), Ciprofloxacin (CIP 5; μg), Sulphathiazole Trimethoprim (SXT; 1.25/23.75 μg) and Amoxicillin(AML;10 μg) were applied on to the media using a disc dispenser and incubated at 370C for 16–18 hours. Using a digital caliper, the zone of inhibition was measured and interpreted as susceptible, intermediate, and resistant using the CLIS [14].
Data analysis
Statistical analysis of the prevalence, Antibiotic resistance rates of Escherichia coli were coded and then entered into an Excel of Microsoft 2016. Descriptive statistics were used to analyze data using Chi- square test, frequency and percentages.
Results
In the current study total of 223 samples were collected 71 Escherichia coli isolated by bacteriological from poultry and poultry environments. All identified isolates were further confirmed by MALDI TOF and finally subjected to AST. The bacteriological methods, MALDI TOF MS, and AST for identification of E. coli isolates are summarized in (Table 1). In this study, 71 (31.83 %) positive E. coli isolates out of the total samples analyzed with MALDI TOF MS identification (Table 2). Risk factors were analyzed with descriptive statistics from all four risk factors. The difference in the occurrence of E. coli among age and different sample types was not statistically significant (P>0.05) in the study. A statistically significant difference in the E. coli prevalence (P less than
The antimicrobial resistant pattern of E. coli isolates from poultry and environmental samples has been in (Table 4). The resistance pattern of E. coli for 12 antibiotics test was 62(87.3%) Amoxicillin, 44(62%) Tetracycline, and 39(54.9%) Streptomycin with their percent respectively. Multi-drug resistance E. coli isolate is shown in (Table 5). Some isolate was sensitive to 60(84.5%) Sulphamethoxazole-Trimethoprim, 57(80.3%) Norfloxacin, 56(78.9 %) Ceftriaxone, and 53(74.6%) Meropenem respectively. Intermediate antibiotics were observed 32(45.1 %) Sulfonamides, and 23(32.4%) Gentamicin for Escherichia coli isolate.
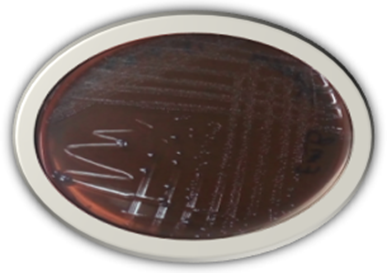
Figure 2: E. coli colony on EMB agar shows a grayish metallic sheen
Table 1: E. coli detection using different tests and methods.
| Detection method | Test | Reaction | No of positive isolate |
| Culture | EMB | Metallic sheen | 71 |
| Gram stain | Gram Negative | 71 | |
| Shape | Small rod | ||
| Color | Pink | ||
| Biochemical | Indole | + | 71 |
| Methyl red | + | 71 | |
| Voges Proskauer | - | 71 | |
| Citrate | _ | 71 | |
| MALDI TOF | Automated | + | 71 |
| AST | Disk diffusion | 71 |
Table 2: Some Escherichia coli isolate confirmed by MALDI TOF MS.
| Lab code | Matched Pattern | Score Value | NCBI Identifier |
| 15429 | Escherichia coli DH5alpha BRL | 2.29 | 562 |
| 15410 | Escherichia coli DH5alpha BRL | 2.24 | 562 |
| 15354 | Escherichia coli DH5alpha BRL | 2.12 | 562 |
| 15371 | Escherichia coli DH5alpha BRL | 2.31 | 562 |
| 15403 | Escherichia coli DH5alpha BRL | 2.14 | 562 |
| 15430 | Escherichia coli DH5alpha BRL | 2.15 | 562 |
| 15419 | Escherichia coli DSM 682 DSM | 2.33 | 562 |
| 15431 | Escherichia coli DSM 682 DSM | 2.46 | 562 |
Table 3: Risk factors that illustrate E. coli distribution within poultry and its environment
| Variable | Classification | No. of Samples | No. of Positive | Percentage of Positive | Chi-Square | P-value |
| Age | Young Adult | 34 | 8 | 3.86% | ||
| 173 | 57 | 27.53% | 0.77381 | 0.379 | ||
| Kebele | Girar | 32 | 9 | 4.03% | 21.106 | 0.0001 |
| Legeberi | 76 | 19 | 8.52% | |||
| Walagaho | 25 | 18 | 8.07% | |||
| Dabe | 90 | 25 | 11.21% | |||
| Sample type | Cloacae | 207 | 65 | 29.14% | 0.25457 | 0.8805 |
| Feed | 8 | 3 | 1.34% | |||
| Water | 8 | 3 | 1.34% | |||
| Farms | 1 | 32 | 9 | 4.03% | 26.991 | 0.002613 |
| 2 | 27 | 8 | 3.58% | |||
| 3 | 2 | 1 | 0.44% | |||
| 4 | 26 | 4 | 1.79% | |||
| 5 | 22 | 8 | 3.58% | |||
| 6 | 11 | 8 | 3.58% | |||
| 7 | 13 | 9 | 4.03% | |||
| 8 | 23 | 7 | 3.13% | |||
| 9 | 29 | 4 | 1.79% | |||
| 10 | 20 | 5 | 2.24% | |||
| 11 | 18 | 8 | 3.58% |
Table 4: Antibiotic susceptibility pattern of 71 E. coli isolates from samples of poultry sources
| Antimicrobial disk | Conc. | Result and interpretation (%) | ||
| S | I | R | ||
| Ciprofloxacin | CIP-5μg | 41(57.7 %) | 12(16.9%) | 18(25.4%) |
| Ceftriaxone | CRO-30μg | 56(78.9 %) | 12 (16.9 %) | 3 (4.2%) |
| Tetracycline | TE-30μg | 27(38.0 %) | 0(0.0%) | 44(62.0%) |
| Amoxicillin+ Cluvanate acid | AMC-30μg | 22(31.0%) | 18(25.4%) | 31(43.7%) |
| Trimethoprim+ Sulphamethoxazole | SXT-25μg | 60(84.5%) | 1(1.4%) | 10(14.1%) |
| Gentamcin | CN-10μg | 40(56.3%) | 23(32.4%) | 8(11.3%) |
| Ampicillin | AMP-10μg | 28(39.4%) | 13(18.3%) | 26(36.6%) |
| Norfloxacin | NOR-10 μg | 57(80.3%) | 3(4.2%) | 11(15.5%) |
| Streptomycin | S-10 μg | 12(16.9%) | 20(28.2%) | 39(54.9%) |
| Sulfonamides | S3-300 | 12(16.9%) | 32(45.1 %) | 27(38 %) |
| Meropenem | MEM-10μg | 53(74.6%) | 15(21.1%) | 3(4.2%) |
| Amoxicillin | AML/AX-2 μg | 1(1.1%) | 8(11.3%) | 62(87.3%) |
Table 5: Summary of antimicrobials Multi drug resistance E. coli isolate
| S/No | No.of Antibiotics disk | No.of Resistant isolates | % Resistants’ isolates |
| 1 | 8 | 2 | 2.81% |
| 2 | 7 | 5 | 7.04% |
| 3 | 6 | 8 | 11.26% |
| 4 | 5 | 16 | 22.53% |
| 5 | 4 | 9 | 12.67% |
| 6 | 3 | 16 | 22.53% |
| 7 | 2 | 9 | 12.67% |
| 8 | 1 | 6 | 8.45% |
| Total | 71 | 100% | |
Discussion
E. coli is the predominant bacteria associated with bacterial infection in birds. These organisms are known to result in severe poultry health problems, leading to mortality, reduced production, and increased expense in preventing and treating the disease. The prevalence of E. coli 29.14 % in cloacae samples in the present study was lower than the previous report in distribution of Escherichia coli in different samples from poultry and poultry environments in Bangladesh [3].The current bacteriological identification method was in agreement with the [15], who conducted tests of Indole, Methyl red, Voges Proskauer reaction and Simmons citrate for biochemical identification. There are similarities between the methods used by [16] in this study who isolates E. coli biochemically and further confirmed using matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI-TOF/MS) from broiler and layer chickens in Mwanza and Arusha regions in Tanzania.
In most countries, chicken is raised with a wide variety of antimicrobials to promote growth and production, and cure infectious disease[17]. Because of the prevalent use of antibiotics, antimicrobial resistance in chickens is a problem in developing nations. In accordance with our study, several E. coli isolates were resistant to the antibiotics 39(54.9%) streptomycin, 44(62%) tetracycline, and 62(87.3%) amoxicillin. The current study found that resistance rates were lower than those reported in Malaysia[18].Improper antimicrobial treatment promotes the emergence and spread of antimicrobial-resistant bacteria among animals. The emergence of multi-drug resistance (MDR) to antimicrobial agents may lead to increased morbidity, mortality, and treatment costs. Multi-drug resistance was observed in this study for more than three or more resistant were detected. In the current investigation higher MDR among E. coli isolates may be a result of chickens receiving excessive doses of antibiotics as prophylactic antibiotic treatments or feed additives. Among the findings that were most similar to the current findings were those of [19], who found that Escherichia coli isolates from Bangladesh had a very high rate of tetracycline resistance, and those of [18], who found that Escherichia coli isolates from poultry farms in North Vietnam had a similar level of resistance. Streptomycin, ampicillin, and tetracycline resistance of a similar nature were noted in Ethiopia[15].
Another important finding was some E. coli isolate was sensitive to 60(84.5%) Trimethoprim Sulphamethoxazole, 57(80.3%) Norfloxacin, 56(78.9 %) Ceftriaxone, and 53(74.6%) Meropenem, 41(57.7 %) ciprofloxacin respectively. In contrast to our findings higher resistance was also recorded for trimethoprim-sulfamethoxazole (89.2%), and ciprofloxacin (68.6%). The high resistance could be because of the lower prices for these antimicrobial agents and also the availability of the antimicrobial agents in Nigeria which make the poultry farmers to easily afford [16].
Conclusion
This study found that Escherichia coli isolated from chickens in Sendafa town were resistant to three or more three antimicrobial agents observed. This a high prevalence of E. coli and antimicrobials resistance to commonly used antibiotics including Amoxicillin, Tetracycline, and Streptomycin in poultry were found. The highest numbers of E. coli resistant to the most of antimicrobials tested as well as the strains with multi drug resistant pattern. These results revealed that chicken production possessing drug resistant to E. coli could be a potential hazard to consumers. Due to the indiscriminate exploitation of antimicrobial agents, such a high occurrence of multi drug resistance may occur. Based on the above finding the following recommendation were forwarded, therefore, In chicken farms, it's critical to follow hygienic procedures, apply antimicrobial agents properly, and take sensible biosecurity precautions. Sustained antimicrobial surveillance frequently and continuous monitoring of chicken production at poultry farms and markets. Excess or abuse use of antibiotics should be reduced. Further study should be conducted to investigate multi drug resistant in E. coli isolates from poultry farms and their environment.
Acknowledgement
The authors are appreciative to all the technical support offered by Bacteriology Laboratory staff of Animal Health Institute (AHI).
Author Contributions
E.M.H. conceived the study design and sample collection. AAU also performed laboratory analysis, generated data, and prepared the original draft. E.M.H. substantively edited the manuscript.
Conflicts of Interest
Author declares that there is no conflict of interest in publishing manuscript
References
- J. Ribeiro et al., (2023). Antibiotic Resistance among Gastrointestinal Bacteria in Broilers: A Review Focused on Enterococcus spp. and Escherichia coli, Animals, 13(8):1-29.
Publisher | Google Scholor - S. Ramos et al., (2020). Escherichia coli as commensal and pathogenic bacteria among food-producing animals: Health implications of extended spectrum β-lactamase (ESBL) production. Animals, 10(12):1-15.
Publisher | Google Scholor - M. A. Akond, S. M. R. Hassan, S. Alam, and M. Shirin. (2009). Antibiotic resistance of escherichia coli isolated from poultry and poultry environment of bangladesh, Am. J. Environ. Sci., 5(1):47-52.
Publisher | Google Scholor - D. Kathayat, D. Lokesh, S. Ranjit, and G. Rajashekara. (2021). Avian pathogenic escherichia coli (Apec): An overview of virulence and pathogenesis factors, zoonotic potential, and control strategies. Pathogens, 10(4):1-32.
Publisher | Google Scholor - J. Kim and J. Ahn. (2022). Emergence and spread of antibiotic-resistant foodborne pathogens from farm to table. Food Sci. Biotechnol., 31(12):1481-1499.
Publisher | Google Scholor - B. Aslam et al. (2021). Antibiotic Resistance: One Health One World Outlook, Front. Cell. Infect. Microbiol., 11:1-20.
Publisher | Google Scholor - W. C. Reygaert. (2018). An overview of the antimicrobial resistance mechanisms of bacteria, 4:482-501.
Publisher | Google Scholor - H. Shaib, P. Aoun, A. Ghaddar, H. Al Labadi, and Y. Obeid. (2023). Multidrug Resistance and Plasmid Profiles of Escherichia coli Isolated from Lebanese. Broiler Farms.
Publisher | Google Scholor - L. Montoro-Dasi, A. Villagra, S. Sevilla-Navarro, M. T. Pérez-Gracia, S. Vega, and C. Marin. (2021). Commensal escherichia coli antimicrobial resistance and multidrug-resistance dynamics during broiler growing period: Commercial vs. improved farm conditions. Animals, 11(4),1-11.
Publisher | Google Scholor - Ababa. (2021). National Meteorological Agency Revised Meteorological Station Network Master Plan Revised Meteorological Station Network Master Plan Preparation Technical Committee.
Publisher | Google Scholor - S. Mudenda et al. (2023). Antimicrobial resistance profiles of Escherichia coli isolated from laying hens in Zambia: implications and significance on one health. JAC-Antimicrobial Resist., 5(3):1-10.
Publisher | Google Scholor - C. G. Clark et al. (2013). Evaluation of MALDI-TOF mass spectroscopy methods for determination of Escherichia coli pathotypes, J. Microbiol. Methods, 94(3)180-191.
Publisher | Google Scholor - J. Hudzicki, Kirby-Bauer (2009). Disk Diffusion Susceptibility Test Protocol Author Information, Am. Soc. Microbiol., 1-13.
Publisher | Google Scholor - R. A. Ibrahim et al. (2019). Erratum: Antimicrobial resistance surveillance in Ethiopia: Implementation experiences and lessons learned. African Journal of Laboratory Medicine, 8:1.
Publisher | Google Scholor - V. Medicine. (2012). Resistance Pattern Of Fecal Escherichia Coli In Selected Broiler Farms Of Eastern Hararghe Zone, Ethiopia. Sampling Site Sample Size Determination Sample Collection Isolation and Identification, 188-194,
Publisher | Google Scholor - R. W. Kiiti, E. V Komba, P. L. Msoffe, S. E. Mshana, M. Rweyemamu, and M. I. N. Matee. (2021). Antimicrobial Resistance Profiles of Escherichia coli Isolated from Broiler and Layer Chickens in Arusha and Mwanza, Tanzania, 2021.
Publisher | Google Scholor - J. J. Carrique-mas. (2017). Antimicrobial Resistance in Bacterial Poultry Pathogens: A Review, 4:1-17.
Publisher | Google Scholor - S. Ibrahim et al. (2021). Prevalence of Antimicrobial Resistance (AMR) Salmonella spp. and Escherichia coli Isolated from Broilers in the East Coast of Peninsular Malaysia.
Publisher | Google Scholor - M. Ajijur et al. (2019). Susceptibility and Multidrug Resistance Patterns of Escherichia coli Isolated from Cloacal Swabs of Live Broiler Chickens in Bangladesh, 1-9.
Publisher | Google Scholor