Research Article
Effects of Major, Minor and Trace Element Concentrations in Normal and Cancerous Prostate Tissues on The Absorbed Dose Heterogeneity During Brachytherapy With 125I Sources
- Vladimir Zaichick *
Radionuclide Diagnostics Department, Medical Radiological Research Centre, Obninsk, Russia.
*Corresponding Author: Vladimir Zaichick, Radionuclide Diagnostics Department, Medical Radiological Research Centre, Obninsk, Russia.
Citation: : Zaichick V. (2024). Effects of Major, Minor and Trace Element Concentrations in Normal and Cancerous Prostate Tissues on The Absorbed Dose Heterogeneity During Brachytherapy With 125I Sources, Journal of BioMed Research and Reports, BioRes Scientia Publishers. 4(5):1-11. DOI: 10.59657/2837-4681.brs.24.079
Copyright: © 2024 Vladimir Zaichick, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: March 14, 2024 | Accepted: March 28, 2024 | Published: April 05, 2024
Abstract
Background: The aim of this study was to determine the dosimetric impact of 69 major, minor and trace elements present in normal or cancerous human prostate tissues, when 125I sources are used in prostate cancer brachytherapy.
Methods: Monte Carlo simulation methods were used for dose distribution calculations. The effect of individual chemical elements on the dose distributions was studied in prostate tissue irradiated by the low-energy photons of an 125I source. The dose differences between cancerous and normal (healthy) prostate tissues were calculated in a single-source geometry.
Results: It was found that for the brachytherapy with an 125I source the bulk elements such as H, C, N, and O, taken together, have the biggest effect on the dose profile in both normal and cancerous prostate tissues. The relative input of photon interactions with H, C, N, and O to the absorbed dose increased with increasing distance from the 125I source, while the relative input of all other chemical elements in dose decreased. The total absorbed dose in cancerous tissues was somewhat lower than in normal tissue and the biggest difference between absorbed doses was located close to the 125I source’s surface and did not exceed 0.9%.
Conclusions: The differences in content of minor and trace elements in normal and cancerous prostate tissues did not play a significant role in the absorbed dose during brachytherapy. This study underlines the need for further investigation into accurate determination of the bulk element composition of tissues irradiated with low-energy brachytherapy for prostate cancer using 125I sources.
Keywords: prostate cancer brachytherapy with 125i source; monte carlo absorbed dose distribution; chemical elements of prostate; dose homogeneity
Introduction
Prostate cancer (PCa) is an important scientific, medical, and social problem. Globally, PCa is the most commonly diagnosed cancer in men [1,2]. PCa incidence rates are highly variable worldwide, but PCa is particularly common in developed countries. Localized PCa can be cured by a variety of treatment options including radical prostatectomy. A satisfactory alternative to prostatectomy, or surgical removal of the affected prostate, is brachytherapy (BT) because it reduces the risk of developing long-term side effects such as impotence and the urinary problems that are commonly observed after prostatectomy. Due to its excellent long-term treatment outcomes BT has become a mainstream treatment option for low-, intermediate-, and high-risk patients with PCa. Thus, BT can be a treatment option for patients suffering from different concomitant diseases, especially heart disease, which is a major contraindication for general anesthesia and hence for radical prostatectomy, and also BT can be used in patients who do not wish to undergo surgery due to personal preferences [3,4]. BT is a form of radiation therapy which involves implantation of radioactive sources directly into the prostate tumor. By ensuring the radiation from the radioinuclides is close to malignant cells, there is much less damage to the surrounding healthy tissue, in comparison with external-beam radiotherapy. Permanent or temporary implantation of the sources of radiation comprise the two different kinds of interstitial BT. Because the source’s radiation has a finite life, in permanent implantation the radioactive sources remain in the cancerous prostate and desired radiation dose is received during source’s life. In temporary implantation, more powerful radioactive sources are removed after the desired radiation dose is received by the cancerous prostate. BT can also be characterized by the rate of radiation dose. The International Commission on Radiation Units and Measurements refers to a dose rate of 40 to 200 cGy per hour (cGy/h) as a low dose rate (LDR), 200 to 1200 cGy/h as a moderate dose rate, and greater than 1200 cGy/h as a high dose rate (HDR) [5].
An increasingly popular form of radiotherapy in PCa is low-energy photon (less than 50 50 keV) BT with a transperineal permanent radioactive seed implantation and, particularly, with an125I seed (Eγ=35keV, T1/2=59.8 days). Thus, the source becomes effectively inactive after a moderate time after delivery of the required dose. Photons with energy ≤35keV from the radionuclide 125I interact with the prostate’s cells primarily through the photoelectric effect, a process which is highly dependent on the chemical element composition of the irradiated tissue. The Monte Carlo (MC) simulation method is a powerful instrument for absorbed radiation dose calculations due to its ability to simulate accurately low-energy photon interactions in realistic geometries. MC simulation methods require knowledge of the nature and distribution of the target atoms (i.e., tissue density with its chemical element composition). The chemical elements forming human soft tissues can be divided into three groups. The first group of elements comprises hydrogen (H), carbon (C), nitrogen (N), and oxygen (O) and they are the major, or bulk, elements, forming the basis of soft tissues. Each of these elements has a content with fractional mass greater than 1% and all together account for about 99% of the total soft tissue mass. The second group of elements with higher atomic numbers (Z), such as sodium (Na), sulfur (S), phosphorus (P), chlorine (Cl) and potassium (K) are ‘minor elements’ of soft tissue and have fractional mass levels between about 0.1% and below 1%. The other chemical elements of soft tissue have trace levels (<0>
A ten-year-old study [6] has demonstrated that uncertainties and variations in the H, C, N, and O human tissue composition could have a significant effect on MC dose distributions. In that work, compositions were varied according to population distributions reported in 1986 by Woodard and White [7], with no special attention paid to the significance of minor and trace elements. There is only one study, to our knowledge, where MC dose calculations were used to investigate the dosimetric effect of 5 minor and 9 trace elements present in normal or cancerous prostates [8]. In this study the effect of individual elements with atomic numbers Z = 17–30 was studied in prostates irradiated by low-energy brachytherapy sources, including 125I. A potentially significant difference in the dose distribution between cancerous and healthy (normal) prostate tissues (of 4% or more) was found. For MC calculations the variation in the content of chemical elements between healthy and cancerous tissue in the prostate was taken from Kwiatek et al 2005 study [9]. However, the data of chemical element concentration presented in this study conflict with the overwhelming corroborating clinical and experimental evidence that has been amassed from numerous reports over the past approximately 60 years [10]. Because the difference in chemical element concentrations between healthy and cancerous prostate tissues was shown to alter the dose distributions, further MC studies with a more accurate prostate composition are needed to understand further this dose variation. In our previous comprehensive studies, the concentration of 66 minor and trace elements in normal and affected prostate tissues were investigated using the most powerful analytical methods such as energy dispersive X-ray fluorescent analysis (EDXRF), instrumental neutron activation analysis (INAA), inductively coupled plasma atomic emission spectrometry (ICP-AES), and inductively coupled plasma mass spectrometry (ICP-MS) [11-55]. Moreover, systematic reviews of the chemical element contents in the normal, hyperplastic, and cancerous prostate glands were done and published [56-58]. It was found using our results and literature data that chemical element composition of normal prostate tissue significantly differs from that of pathological prostates, particularly, cancerous glands. Malignant prostate tumors are a mixture of non-affected (intact) and cancerous tissues. Because dose calculations for low energy photons are sensitive to tissue heterogeneities, the present study was performed to investigate the importance of major, minor, and trace elements in brachytherapy with 125I sources in healthy and cancerous human prostate tissues using MC simulation techniques.
Materials and Methods
Composition of Tissues
The mass fractions of major chemical elements H, C, N, and O of the human prostate used in the study was taken from a frequently cited Report 46 of International Commission on Radiation Units and Measurements (ICRU, 1992) and Publication 89 of International Commission on Radiation Protection (ICRP, 2003) [59,60]. While it has been shown that uncertainties in the composition of H, C, N, and O can affect the results of low-energy brachytherapy [6], the objective of this study was the investigation of the effects of minor and trace element variations. Because no recent comprehensive study into H, C, N, and composition in normal and cancerous prostate tissues could be found, throughout the study the proportionality between H, C, N and O in each tissue was presumed to be fixed, while the minor and trace component varied in accordance with our findings and data obtained from reviews [11-58] (Table 1). In addition to this, the density of normal and cancerous prostate tissue was also presumed to be constant – 1040 kg/m3.
Table 1: Mass fractions of major, minor and trace elements (mg/kg wet mass basis) of human normal and cancerous prostate tissues relevant to low-energy photon brachytherapy.
| Element | Norm | Cancer | Element | Norm | Cancer | Element | Norm | Cancer |
| H | 105000 | 105000 | Eu | 0.00012 | 0.00211 | Pt | 0.00012 | 0.00284 |
| C | 256000 | 256000 | Fe | 24.4 | 44.5 | Rb | 2.89 | 2.37 |
| N | 25000 | 25000 | Ga | 0.0155 | 0.202 | Re | 0.00020 | 0.0075 |
| O | 602000 | 602000 | Gd | 0.00061 | 0.00128 | S | 2174.3 | 2301.2 |
| Ag | 0.008 | 0.0712 | Hf | 0.0042 | 0.104 | Sb | 0.0085 | 0.138 |
| Al | 6.95 | 99.5 | Hg | 0.0103 | 0.0333 | Sc | 0.0059 | 0.00303 |
| As | 0.005 | 0.225 | Ho | 0.00012 | 0.00049 | Se | 0.149 | 0.153 |
| Au | 0.00083 | 0.0087 | Ir | 0.00009 | 0.00196 | Si | 20.7 | 80.7 |
| B | 0.197 | 3.75 | K | 2355 | 2367 | Sm | 0.00055 | 0.00276 |
| Ba | 0.29 | 8.19 | La | 0.017 | 0.238 | Sn | 0.0641 | 0.384 |
| Be | 0.00019 | 0.00401 | Li | 0.00823 | 0.075 | Sr | 0.51 | 1.78 |
| Bi | 0.0061 | 0.51 | Lu | 0.00005 | 0.00018 | Ta | 0.00113 | 0.024 |
| Br | 7.12 | 29.1 | Mg | 213 | 95.6 | Tb | 0.00008 | 0.00022 |
| Ca | 502 | 186 | Mn | 0.269 | 2.02 | Th | 0.00068 | 0.0149 |
| Cd | 0.225 | 0.12 | Mo | 0.0553 | 0.0717 | Ti | 0.57 | 2.16 |
| Ce | 0.0064 | 0.0289 | Na | 2200 | 2074 | Tl | 0.00028 | 0.0066 |
| Cl | 2754 | 2754 | Nb | 0.00115 | 0.0012 | Tm | 0.00005 | 0.00014 |
| Co | 0.0093 | 0.0092 | Nd | 0.00286 | 0.0122 | U | 0.00133 | 0.00198 |
| Cr | 0.108 | 0.626 | Ni | 0.647 | 2.01 | V | 0.0469 | 0.45 |
| Cs | 0.00663 | 0.0111 | P | 1529 | 1899 | Y | 0.0034 | 0.0099 |
| Cu | 1.92 | 4.65 | Pb | 0.479 | 0.495 | Yb | 0.0003 | 0.0004 |
| Dy | 0.00061 | 0.00209 | Pd | 0.00152 | 0.0405 | Zn | 205 | 37.8 |
| Er | 0.00031 | 0.00086 | Pr | 0.00073 | 0.00291 | Zr | 0.0073 | 0.61 |
| ∑ | 1000000,0 | 1000000,0 |
Data for H, C, N, and O in prostate tissue taken from ICRU and ICRP reports,59,60 and for ME and TE from published reviews [56-58].
Simulation Technique
The simulation was made using GEANT4 framework (version 10.7) [61,62]. Two separate simulation series were carried out for each type of tissue. Concentric spherical layers with thickness of 0.1 mm located inside a spherical phantom with diameter of 104 mm were used as the detectors. The inner radius of the first layer R_min was 4 mm and the outer radius of the last layer R_max was 50 mm. In each simulation series, the phantom was filled with a homogeneous tissue, the composition of which is given in Composition of Tissues. The point isotropic source was located at the center of the phantom. The spectral characteristics of the source corresponded to the initial spectrum of the γ- and X-rays from the 125I radioactive decay. The yields and energies of photons were taken from the ENDF-B/VIII.0 library (Table 2). This approach was used because there are many clinical sources for brachytherapy based on the 125I [63-68]. These sources have different dosimetric characteristics due to using the different geometry and materials [63-67]. The approach used makes it possible to generalize the obtained results to all types of sources. To simulate the interaction of the radiation with matter, the library for modeling low-energy electromagnetic interactions G4EmLivermore Physics was used [69].
Table 2: Spectrum of photons emitted during the radioactive decay of 125I.
| Photon energy (keV) | Yield (%) |
| 3.36 | 1.00 |
| 3.76 | 7.35 |
| 4.08 | 6.20 |
| 27.19 | 40.11 |
| 27.46 | 74.66 |
| 30.94 | 6.87 |
| 30.99 | 13.33 |
| 31.67 | 1.42 |
| 31.68 | 2.80 |
| 35.49 | 6.68 |
During simulation, the depth profiles of deposited energy, which is directly proportional to the absorbed dose, were obtained for each type of tissue for the cases when the separate ME or TE were removed from the tissue composition. Additionally, the profiles of deposited energy were calculated for two cases - when all ME and TE were taken into account and all ME and TE were removed excepting H, C, N and O. The tissue density and the ratio of concentration for remaining elements were the same. The change of absorbed dose in each case was evaluated using the equation 1:

where – the integral of the deposited energy
– the integral of the deposited energy calculated when this or that chemical element was removed;
calculated when this or that chemical element was removed;  – the integral of the deposited energy
– the integral of the deposited energy calculated for the initial elemental composition (BE+ME+TE). The profiles of deposited energy obtained for normal and malignantly transformed prostate tissue, taking into account the influence of all chemical elements, were also compared according to equation 1.
calculated for the initial elemental composition (BE+ME+TE). The profiles of deposited energy obtained for normal and malignantly transformed prostate tissue, taking into account the influence of all chemical elements, were also compared according to equation 1.
Results
The relative difference between the results of the MC simulations obtained for normal and cancerous prostate tissue were plotted against the radial distance from the center of source with radionuclide 125I (Figure 1). The calculations were done using the chemical element compositions of normal and cancerous prostate tissue presented in Table 1 and the tissue density 1040 kg/m3.
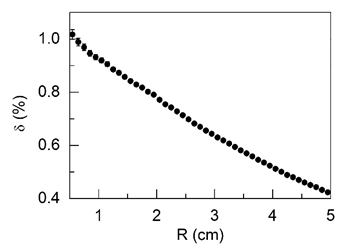
Figure 1: Relative reduction δ (see equation 1 in the text) of the absorbed dose in cancerous tissue compared to normal prostate tissue depending on the radial distance from the center of the 125I source (R).
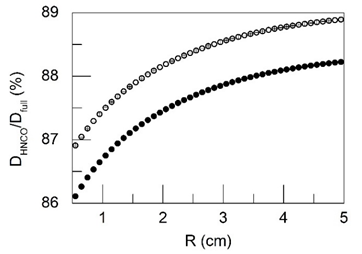
Figure 2 presents the ratio of the integral of absorbed doses obtained for the case when all ME and TE are removed from the tissue composition (DHNCO) and for the total (BE+ME+TE) tissue composition (Dfull) depending on the radial distance from the center of the 125I source (R). The data shown in the Figure 2 corresponds to normal and cancerous prostate tissue.
Figure 2: The ratio of the integral of absorbed doses obtained for the case when all minor and trace elements are removed from the tissue composition (DHNCO) and for the total tissue composition (Dfull) depending on the radial distance from the center of the 125I source (R). The data shown in the figure corresponds to normal (●) and cancerous (○) prostate tissue.
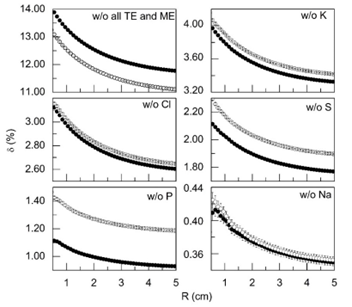
Figure 3 and 4 shows the relative change in the integral of the absorbed dose δ (%) depending on the radial distance R(cm) from the center of the source 125I when individual chemical elements are removed from the tissue composition. The data are given for normal and cancerous prostate tissue. Figure 3 also depicts the relative change of the dose when all chemical elements are removed excepting H, N, C and O.
Figure 3: The relative decrease in the integral of the absorbed dose δ (see equation 1 in the text) depending on the radial distance from the center of the 125I source (R), associated with the exclusion of separated chemical elements from the tissue composition: - w/o all TE and ME - tissue composition without all trace and minor elements, -w/o K, w/o Cl, w/o S, w/o P, w/o Na - tissue composition includes all major, minor and trace elements, with the exception of K, Cl, S, P and Na, respectively. The data shown in the figure corresponds to normal (●) and cancerous (○) prostate tissue.
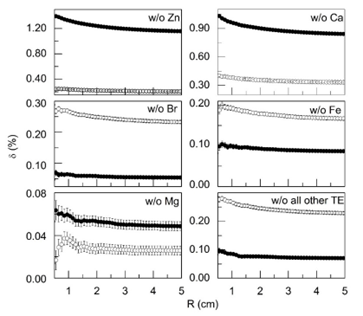
Figure 4: The relative decrease in the integral of the absorbed dose δ (see equation 1 in the text) depending on the radial distance from the center of the 125I source (R), associated with the exclusion of separated trace elements from the tissue composition: -w/o Zn, w/o Ca, w/o Br, w/o Fe, w/o Mg - tissue composition includes all major, minor, and trace elements with exception Zn, Ca, Br, Fe, and Mg, respectively, -w/o all other TE - tissue composition includes all major and minor chemical elements, and only such trace elements as Zn, Ca, Br, Fe, and Mg. The data shown in the figure corresponds to normal (●) and cancerous (○) prostate tissue.
Discussion
Malignant tumors of prostate are a strong mixture of non-affected and cancerous tissues. In present study, it was supposed that the composition of non-affected tissues in prostate malignant tumors was just the same as the composition of normal tissue of gland. The mass fractions of 69 chemical elements contents in normal and cancerous prostate tissues (Table 1) presented the most reliable information on the subject because results for four bulk elements H, C, N, and O were taken from the official reports of International Committees (ICRU, 1992 and ICRP, 2003) and results for other 65 ME and TE, obtained by us, were in a good agreement with median values of reported data [56-58]. The main uncertainties of our calculations connected with four bulk elements H, C, N, and O contents in cancerous prostate tissues. Because of the lack of data reported in literature it was supposed by us that mass fractions of H, C, N, and O in cancerous prostate tissues did not differ from those in normal tissue of gland. In addition to this, the density of each tissue was also kept constant. The relative dose difference between cancerous and healthy prostate tissues was calculated using equation 1. The results are shown in Figure 1. If the above assumptions are correct, then it should be concluded that differences in the content of ME and TE in normal and cancerous tissues of the prostate gland do not play a significant role in dose formation in BT. Absorbed doses in cancerous tissues are somewhat lower than in normal tissues, and the largest relative difference between absorbed doses is located near the surface of the 125I source and does not exceed 1%. Such small effect of differences in ME and TE contents in normal and cancerous prostate tissue can be explained by the different direction of changes in the content of chemical elements. For example, the mass fraction of Na, Mg, Ca, and Zn are lower, while P, S, and Fe are higher in cancerous tissue in comparison with normal prostate tissue (Table 1).
As can be seen from the Figure 2 and 3, ME and TE have a non-negligible (up to 13-14%) effect on the dose in prostate tissues irradiated with low-energy photons of 125I source. The results presented in Figure 3 make it possible also to evaluate the radial profiles of the relative decrease in the integral of the absorbed dose δ (%) associated with the exclusion of individual ME such as K, Cl, S, P and Na from the composition of the normal and cancerous prostate tissues. Among these ME, the biggest influence on dose formation in normal prostate tissue at distance 0.5 cm from the source’s center had the photon’s interaction with K (4.0%) and Cl (3.1%), while the lowest value–the photon’s interaction with Na (0.4%).In cancerous prostate tissue, the biggest values of relative dose change at distance 0.5 cm from the source’s center had also the photon’s interaction with K (relative difference up to 4.1%) and Cl (3.15%), while the lowest values also had the interaction with Na(0.42%). The difference between the relative dose change in normal and cancerous tissues associated with these ME at the distance of 0.5 cm from the source was -0.3% for P, -0.18% for S, -0.09% for K, -0 .03% for Cl and -0.01% for Na. In total, variations in the content of ME in normal and cancerous tissue provided a relative difference in the absorbed dose at the distance of 0.5 cm from the source of 125I equal to -0.61%. The mass fractions of Mg, Ca, and Zn in normal prostate tissue are 213, 502, and 205 mg/kg wet mass basis, respectively (Table 1). These values are highest among mass fractions of all other TE of normal prostate tissue. The mass fractions of Fe (24.4 mg/kg wet mass basis) and Br (7.12 mg/kg wet mass basis) are almost one and two order of magnitude lower in comparison with Ca, however these TE have higher photon’s absorption coefficients. This was one of the reasons for choosing these TE for calculations as well. A significant difference in the mass fractions of these TE in normal and cancerous tissues of the prostate was another reason for their choice.
The results presented in Figure 4 make it possible to evaluate the radial profiles of the relative decrease in the integral of the absorbed dose δ (%) associated with the exclusion of TE such as Br, Ca, Fe, Mg, and Zn, as well as all other 55 TE taken together from the composition of the normal and cancerous prostate tissues. As can be seen from the Figure 4, among TE of normal prostate tissue the biggest values of relative change in dose at distance 0.5 cm from the source’s center had the photon’s interaction with Zn (1.4%), while the lowest value – the photon’s interaction with Mg (0.06%). The maximal change in dose other 55 TE of normal prostate tissue, taken together, did not exceed 0.1%. In cancerous prostate tissue, the biggest value of relative dose change at distance 0.5 cm from the source’s center had the photon’s interaction with Ca (0.4%), while the lowest value also had the interaction with Mg (0.03%). The maximal change in dose for other 55 TE of normal prostate tissue, taken together, did not exceed 0.28%. The differences between the relative dose change in the normal and cancerous tissues for these TE at the distance from the source of 0.5 cm were 1.15% for Zn, 0.63% for Ca, -0.2% for Br, -0.1% for Fe and -0.03% for Mg. In total, variations in the content of TE in normal and cancerous tissue provided a relative difference in the absorbed dose at the distance of 0.5 cm from the source of 125I equal to 1.45%.
Finally, the relative changes in dose at distance 0.5 cm from the 125I source’s center associated with variations in the content of ME and TE in normal and cancerous tissue equal to (-0.61% +1.45%) = 0.84%. Thus, the biggest difference between the relative dose changes for all 65 ME and TE in normal and cancerous prostate tissue did not exceed 0.9% and agreed well with result presented above in Figure 1. This result contradicts the findings of White et al. [8], based on incorrect data on the content of TE in the prostate gland published by Kwiatek et al. [9]. Our results suggest that the greatest uncertainty in dose heterogeneity stems from the lack of data on differences in BE content between normal and cancerous prostate tissue, as there is evidence to suggest that these differences may be quite significant. For example, it is well known that many malignant tumors are hypoxic, i.e., the oxygen concentration in them is lower than in normal tissue. The successful use of magnetic resonance imaging for the diagnosis of PCa indicates different hydrogen content in the tumor and surrounding tissues. Therefore, further studies should be aimed at precise determination of BE concentrations in normal and cancerous prostate tissue.
Conclusion
Our results obtained using fairly accurate data on the content of ME and TE in normal and cancerous human prostate tissues in MC modeling, led to the conclusion that tissue composition variability does not affect the degree of absorbed dose uniformity in prostate BT by 125I sources. Further study of the H, C, N, and O contents in human normal and cancerous prostate tissues is needed, as well as studies into the potential variability between bulk compositions of different kinds of malignant tumors. Otherwise, the lack of accurate data on BE concentrations in normal and cancerous prostate tissue will contribute to inaccuracy in assessing dose uniformity in BT of PCa.
Acknowledgement
The author is grateful to Dr. Pavel Prusachenko (I.I. Leypunsky Institute of Physics and Energy) for assistance in Monte Carlo simulations.
References
- Sekhoacha M, Riet K, Motloung P, Gumenku L, Adegoke A, et al. (2022). Prostate cancer review: genetics, diagnosis, treatment options, and alternative approaches. Molecules. 27(17):5730.
Publisher | Google Scholor - Rawla P. (2019). Epidemiology of Prostate Cancer. World J Oncol. 10(2):63-89.
Publisher | Google Scholor - Chin J, Rumble RB, Kollmeier M, et al. (2017). Brachytherapy for patients with prostate cancer: American society of clinical oncology/cancer care Ontario joint guideline update. J Clin Oncol. 35(15):1737-1743.
Publisher | Google Scholor - Koukourakis G, Kelekis N, Armonis V, Kouloulias V. (2009). Brachytherapy for prostate cancer: A systematic review. Adv Urol. 327945:1-11.
Publisher | Google Scholor - International Commission on Radiation Units and Measurements (ICRU). (1985). Dose and volume specification for reporting intracavitary therapy in gynecology. Tech. Rep. 38. Bethesda, Md, USA: ICRU.
Publisher | Google Scholor - Landry G, Reniers B, Murrer L, et al. (2010). Sensitivity of low energy brachytherapy Monte Carlo dose calculations to uncertainties in human tissue composition. Med Phys. 37(10):5188-5198.
Publisher | Google Scholor - Woodard HQ, White DR. (1986). The composition of body tissues. Br J Radiol. 59:1209-1218.
Publisher | Google Scholor - White SA, Landry G, van Gils F, Verhaegen F, Reniers B. (2012). Influence of trace elements in human tissue in low-energy photon brachytherapy dosimetry. Phys Med Biol. 57(11):3585-3596.
Publisher | Google Scholor - Kwiatek W M, Banas A, Gajda M, et al. (2005). Cancerous tissues analyzed by SRIXE. J Alloys Compd. 401:173-177.
Publisher | Google Scholor - Costello LC, Franklin RB. (2011). Zinc is decreased in prostate cancer: an established relationship of prostate cancer! J BiolInorg Chem. 16(1):3-8.
Publisher | Google Scholor - Zaichick S, Zaichick V. (2010). Method and portable facility for energy-dispersive X-ray fluorescent analysis of zinc content in needle-biopsy specimens of prostate. X-Ray Spectrom. 39:83-89.
Publisher | Google Scholor - Zaichick S, Zaichick V. (2011). INAA application in the age dynamics assessment of Br, Ca, Cl, K, Mg, Mn, and Na content in the normal human prostate. J Radioanal Nucl Chem. 288(1):197-202.
Publisher | Google Scholor - Zaichick S, Zaichick V. (2011). The effect of age on Ag, Co, Cr, Fe, Hg, Sb, Sc, Se, and Zn contents in intact human prostate investigated by neutron activation analysis. Appl Radiat Isot. 69:827-833.
Publisher | Google Scholor - Zaichick S, Zaichick V. (2011). The Br, Fe, Rb, Sr, and Zn contents and interrelation in intact and morphologic normal prostate tissue of adult men investigated by energy-dispersive X-ray fluorescent analysis. X-Ray Spectrom. 40(6):464-469.
Publisher | Google Scholor - Zaichick S, Zaichick V. (2012). Trace elements of normal, benign hypertrophic and cancerous tissues of the human prostate gland investigated by neutron activation analysis. Appl Radiat Isot. 70:81-87.
Publisher | Google Scholor - Zaichick V, Nosenko S, Moskvina I. (2012). The effect of age on 12 chemical element contents in intact prostate of adult men investigated byinductively coupled plasma atomic emission spectrometry. Biol Trace Elem Res. 147:49-58.
Publisher | Google Scholor - Zaichick S, Zaichick V, Nosenko S, Moskvina I. (2012). Mass fractions of 52 trace elements and zinc trace element content ratios in intact human prostates investigated by inductively coupled plasma mass spectrometry. Biol Trace Elem Res. 149(2):171-183.
Publisher | Google Scholor - ZaichickS, Zaichick V. (2013). Relations of morphometric parameters to zinc content in paediatric and nonhyperplastic young adult prostate glands. Andrology. 1(1):139-146.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2013). INAA application in the assessment of Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se, and Zn mass fraction in pediatric and young adult prostate glands. J Radioanal Nucl Chem. 298(3):1559-1566.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2013). The effect of age on Br, Ca, Cl, K, Mg, Mn, and Na mass fraction in pediatric and young adult prostate glands investigated by neutron activation analysis. Appl Radiat Isot. 82:145-151.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2013). NAA-SLR and ICP-AES application in the assessment of mass fraction of 19 chemical elements in pediatric and young adult prostate glands. Biol Trace Elem Res. 156(1):357-366.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2013). Use of neutron activation analysis and inductively coupled plasma mass spectrometry for the determination oftrace elements in pediatric and young adult prostate. American Journal of Analytical Chemistry. 4:696-706.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2014). Age-related histological and zinc content changes in adult nonhyperplastic prostate glands. Age. 36(1):167-181.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2014). Relations of bromine, iron, rubidium, strontium, and zinc content to morphometric parameters in pediatric and non-hyperplastic young adult prostate glands. Biol Trace Elem Res. 157(3):195-204.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2014). Relations of the Al, B, Ba, Br, Ca, Cl, Cu, Fe, K, Li, Mg, Mn, Na, P, S, Si, Sr, and Zn mass fractions to morphometric parameters in pediatric and non-hyperplastic young adult prostate glands. Biometals, 27(2):333-348.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2014). INAA application in the assessment of chemical element mass fractions in adult and geriatric prostate glands. Appl Radiat Isot. 90:62-73.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2014). Androgen-dependent chemical elements of prostate gland. Androl Gynecol: Curr Res. 2:2.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2014). Use of INAA and ICP-MS for the assessment of trace element mass fractions in adult and geriatric prostate. J Radioanal Nucl Chem. 301(2):383-397.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2014). Determination of trace elements in adults and geriatric prostate combining neutron activation with inductively coupled plasma atomic emission spectrometry. Open Journal of Biochemistry. 1(2):16-33.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2014). Relations of the neutron activation analysis data to morphometric parameters in pediatric and non-hyperplastic young adult prostate glands. Advances in Biomedical Science and Engineering. 1(1):26-42.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2014). The distribution of 54 trace elements including zinc in pediatric and nonhyperplasticyoung adult prostate gland tissues. Journal of Clinical and Laboratory Investigation Updates. 2(1):1-15.
Publisher | Google Scholor - Zaichick V, Zaichick S, Davydov G. (2015). Differences between chemical element contents in hyperplastic and non-hyperplastic prostate glands investigated by neutron activation analysis. Biol Trace Elem Res. 164:25-35.
Publisher | Google Scholor - ZaichickV, Zaichick S. (2015). Differences and relationships between morphometric parameters and zinc content in non-hyperplastic and hyperplastic prostate glands. British Journal of Medicine & Medical Research. 8(8):692-706.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2015). Dietary intake of minerals and prostate cancer: insights into problem based on the chemical element contents in the prostate gland. J Aging Res Clin Practice. 4(3):164-171.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2015). Global contamination from uranium: insights into problem based on the uranium content in the human prostate gland. J Environ Health Sci. 1(4):1-5.
Publisher | Google Scholor - Zaichick S, Zaichick V. (2015). Prostatic tissue level of some androgen dependent and independent trace elements in patients with benign prostatic hyperplasia. Androl Gynecol: Curr Res. 3:3.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2016). The bromine, calcium, potassium, magnesium, manganese, and sodium contents in adenocarcinoma of human prostate gland. J Hematology & Oncology Research. 2(2):1-12.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2016). Trace element contents in adenocarcinoma of human prostate investigated by energy dispersive X-ray fluorescent analysis. Journal of Adenocarcinoma& Osteosarcoma. 1(1):1-7.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2016). Trace element contents in adenocarcinoma of the human prostate gland investigated by neutron activation analysis. Journal of Adenocarcinoma. 1(1):1-10.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2016). Variations in concentration and distribution of several androgen-dependent and -independent trace elements in non-hyperplastic prostate gland tissue throughout adulthood. J Androl Gynaecol. 4(1):1-10.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2016). Chemical elemental content/Calcium ratios in tissues of human hyperplastic prostate gland. Journal of Applied Life Sciences International. 4(4):1-11.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2016). Prostatic tissue levels of 43 trace elements in patients with prostate adenocarcinoma. Cancer and Clinical Oncology. 5(1):79-94.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2016). Levels of 43trace elements in hyperplastic prostate tissues. British Journal of Medicine and Medical Research. 15(2):1-12.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2016). Prostatic tissue level of some major and trace elements in patients with BPH. Jacobs Journal of Nephrology and Urology. 3(1):025.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2016). Age-related changes in concentration and histological distribution of Br, Ca, Cl, K, Mg, Mn, and Na in non-hyperplastic prostate of adults. European Journal of Biology and Medical Science Research. 4(2):31-48.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2016). Variations in concentration and histological distribution of Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se, and Zn in non-hyperplastic prostate gland throughout adulthood. Jacobs Journal of Cell and Molecular Biology. 2(1):011.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2016). Age-related changes in concentration and histological distribution of 18 chemical elements in non-hyperplastic prostate of adults. World Journal of Pharmaceutical and Medical Research. 2(4):5-18.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2016). The Comparison between the contents and interrelationships of 17 chemical elements in normal and cancerous prostate gland. Journal of Prostate Cancer. 1(1):105.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2016). Distinguishing malignant from benign prostate using Br, Ca, K, Mg, Mn, and Na content in prostatic tissue. Integrative Molecular Medicine. 3(3):733-738.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2016). Distinguish between benign and malignant prostate using the trace element content ratios in prostatic tissue as tumor markers. M J Canc. 1(1):006.
Publisher | Google Scholor - Rossmann M, Zaichick S, Zaichick V. (2016). Distinguishing malignant from benign prostate tumors using Br, Fe, Rb, Sr, and Zn content in prostatic tissue. The Journal Prostate Cancer- Clinics in Oncology. 1:1054.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2016). Distinguishing malignant from benign prostate using content of 17 chemical elements in prostatic tissue. Integr Cancer Sci Therap. 3(5):579-587.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2016). Age-related changes in concentration and histological distribution of 54 trace elements in non-hyperplastic prostate of adults. Int Arch Urol Complic. 2(2):019.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2017). Trace element levels in prostate gland as carcinoma’s markers. Journal of Cancer Therapy. 8:131-145.
Publisher | Google Scholor - Zaichick V, Zaichick S. Chemical element contents in normal and benign hyperplastic prostate. Ann Mens Health Wellness. 1(2):1006.
Publisher | Google Scholor - Zaichick V. (2015). The variation with age of 67 macro- and microelement contents in non-hyperplastic prostate glands of adult and elderly males investigated by nuclear analytical and related methods. Biol Trace Elem Res. 168(1):44-60.
Publisher | Google Scholor - Zaichick V. (2017). Differences between 66 chemical element contents in normal and cancerous prostate. Journal of Analytical Oncology. 6(1):37-56.
Publisher | Google Scholor - Zaichick V, Zaichick S. (2019). Comparison of 66 chemical element contents in normal and benign hyperplastic prostate. Asian Journal of Urology. 6(3):275-289.
Publisher | Google Scholor - International Commission on Radiation Units and Measurements (ICRU). (1992). Photon, electron, proton, and neutron interaction data for body tissues. Report 46. Bethesda, MD, USA: ICRU.
Publisher | Google Scholor - International Commission on Radiation Protection (ICRP). (2003). Basic anatomical and physiological data for use in radiological protection: reference values. Publication 89. Elsevier Science Ltd.
Publisher | Google Scholor - Agostinelli S, Allison J, Amako K, et al. (2003). Geant4, a simulation toolkit. Nucl Instrum Methods Phys Res. A506:250-303.
Publisher | Google Scholor - Allison J, Amako K, Apostolakis J, et al. (2016). Recent developments in GEANT4. Nucl Instrum Methods Phys Res. A835:186-225.
Publisher | Google Scholor - Al YasiriAY, Abed HF. (2018). Estimation of Energy Spectrum and Energy Deposition of Photons Emitted from Brachytherapy 125I Seed. Indian Journal of Science and Technology. 11(24):1-5.
Publisher | Google Scholor - Solberg TD, DeMarco JJ, Hugo G, WallaceRE. (2002). Dosimetric parameters of three new solid core I‐125 brachytherapy sources. Journal of Applied Clinical Medical Physics. 3(2):119-134.
Publisher | Google Scholor - Heintz BH, Wallace RE, Hevezi JM. (2001). Comparison of I‐125 sources used for permanent interstitial implants. Med Phys. 28(4):671-682.
Publisher | Google Scholor - Beyer DC, Puente F, Rogers KL, Gurgoze EM. (2001). Prostate brachytherapy: Comparison of dose distribution with different 125I source designs. Radiology. 221(3):623-627.
Publisher | Google Scholor - Saw CB, Meigooni AS, Nath R. (1998). Review of AAPM task group NO. 43 recommendations on interstitial brachytherapy sources dosimetry. Medical Dosimetry. 23(4):259-263.
Publisher | Google Scholor - International Atomic Anergy Agency (IAEA). (2006). Production techniques and quality control of sealed radioactive sources of palladium-103, iodine-125, iridium-192 and itterbium-169, IAEA-TECHDOC-1512. Vienna, Austria: IAEA.
Publisher | Google Scholor - Geant 4 Collaboration. (2022). Physics reference manual, 11.1.
Publisher | Google Scholor