Case Report
Effect of Vitamin C on Subacute Cypermethrin Induced Hematotoxicity and Brain Histopathological Changes in Wistar Rats
1Ezekiel Moses, Department of Research, Innovation and Technology Development, Federal College of Fisheries and Marine Technology, Victoria Island, Lagos, Nigeria.
2Department of Research, Innovation and Technology Development, Federal College of Fisheries and Marine Technology, Victoria Island, Lagos, Nigeria.
*Corresponding Author: Ezekiel Moses, Ezekiel Moses, Department of Research, Innovation and Technology Development, Federal College of Fisheries and Marine Technology, Victoria Island, Lagos, Nigeria.
Citation: Moses E, Amanda E. Izegaegbe. (2024). Effect of Vitamin C on Subacute Cypermethrin Induced Hematotoxicity and Brain Histopathological Changes in Wistar Rats. Clinical Case Reports and Studies, BioRes Scientia Publishers. 6(3):1-6. DOI: 10.59657/2837-2565.brs.24.141
Copyright: © 2024 Ezekiel Moses, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: May 29, 2024 | Accepted: June 20, 2024 | Published: July 10, 2024
Abstract
The study was aimed at evaluating the effects of vitamin C on cypermethrin induced hematological and histological changes in adult Wistar rats. Twenty apparently healthy adult Wistar rats were used for this study. The animals were acclimatized for two weeks before the commencement of the study. The rats were divided randomly into 4 groups of 5 animals each. Animals in group A were dosed with distilled water (DW), while those in group B were given Cypermethrin (CYP) at 30mg/kg. The rats in group C were treated with vitamin C (100mg/kg), while those in group D were administered sequentially with cypermethrin (CYP) (30mg/kg) and 30minutes after vitamin C (100mg/kg) was given. The treatments were administered once daily by oral gavage for 2 weeks and all rats were observed for clinical signs within the test period. Clinical signs were observed within the test period. At the end of the experiment, blood (2mls) was collected directly from the heart and brain samples were also collected using dissecting tools from each group. The rats in group B showed clinical signs of diarrhea, salivation, restlessness, scratching and rubbing their mouth on beddings and rough hair coat with erect hairs. Rats in groups A, C and D showed no clinical signs. The decreased WBC, reduced RBC counts and increased MCHC were ameliorated by treatment with vitamin C in group D. Treatment with vitamin C did not affect the body weight of these animals. Vacuoles were seen in the histopathological sections of the brain of groups B and D, which was more evident in group B, while groups A and C were normal. From this study it is concluded that vitamin C mitigated the hematological and brain histopathological changes induced by cypermethrin probably due to its antioxidant mechanism.
Keywords: cypermethrin; sub-acute; hematological; vitamin c; toxicity
Introduction
Pesticides are beneficial in controlling agricultural pests (including diseases and weeds) and vectors of plant disease, controlling human and livestock disease vectors and nuisance organisms and preventing or controlling organisms that harm other human activities and structures [1]. In primary industry, the application of herbicides to control weeds and insecticides to control invertebrate pests can improve the yield and quality of food and fibre crops [2]. Pesticides are also important tools in the prevention and treatment of termite infestation in buildings. The control of biting insects such as mosquitoes, which can be a nuisance as well as vectors of disease, provides public health benefits and improves the quality of our lives. The use of pesticides in recreational areas such as sporting ovals, golf courses and parks have social, recreational and aesthetic benefits for our community [2]. More so, many exotic organisms such as weeds or feral species of animals can cause harm to the natural environment. Pesticide’s use can also help control environmentally harmful organisms, leading to the protection of native habitats and the maintenance of biodiversity [2]. There is a large range of benefits from different types of pesticides used which includes reduced crop loss resulting from spraying fungicides, but some are less obvious either because they occur in the medium or long term, or are subtle or small incremental benefits distributed over a large area [1]. Though beneficial, they cause harm when accidentally ingested. It is important to prevent these dangers because consumers are prone to ingesting pesticides from residues in food and water [3]. Therefore, pesticide misuse and management of wastes, including pesticides waste are important to prevent or mitigate off-site pesticide risks to soil, water, air, plant, animals and humans from drift and volatilization losses.
Materials and Methods
Experimental Animals
Twenty adult Wistar rats weighing between 150 - 200 g were obtained from the Animal House of the Department of Pharmacology and Toxicology, Faculty of Veterinary Medicine, Usmanu Danfodiyo University Sokoto, Nigeria. They were housed in cages (5 animals per cage) in the Toxicology Laboratory of the Department. The animals were acclimatized for two weeks before the commencement of the study. They were given access to standard rat feed and tap water was provided ad libitum.
Chemical Acquisition and Preparation
Commercial grade cypermethrin (Jiangsu Yangnog Chemical Co. Limited, China) was obtained from reputable Agrochemical Stores in Zaria, Kaduna Nigeria. It was reconstituted to appropriate working concentrations of 100 mg/ml and vitamin C (Archy pharmaceuticals Nigeria, LTD) 100 mg/ ml. Prior to daily administration, 100 mg of vitamin C was reconstituted in distilled water to obtain 100 mg/ml suspension.
Determination of LD50 of Cypermethrin
The first phase involved the use of nine male rats divided into three groups of three rats each (I, II, III). Rats in Group I, II, III were dosed orally with cypermethrin at 1000, 100 and 10mg/kg, respectively. The animals were observed for death over a period of 48 hours. The evaluation proceeded to the second phase which depended on the results obtained from the first phase. In the second phase, three male rats were divided into three groups (Ia, IIa, IIIa) of one animal each and cypermethrin was administered at the dose rate of 150 mg/kg for group Ia, 250 mg/kg for group IIa and 350 mg/kg for group IIIa, respectively. Death was recorded over a period of 48 hours. The mean of the highest dose that caused death and the highest dose which the rats were alive was recorded. The LD50 was calculated based on the dose-response relationship as described by [4].
Animal Treatments
Twenty adult Wistar rats of both sexes, weighing 150 – 200 g were used for this study. The rats were divided at random into 4 groups (A, B, C and D) of 5 rats each. They were marked on their tails with a marker for identification. Animals in group A were administered with distilled water (DW), while those in group B were given cypermethrin (CYP) at 30mg/kg. The rats in group C were treated with Vitamin C (100 mg/kg) while those in group D were administered sequentially with cypermethrin (CYP) (30 mg/kg, ~ 1/10th of the LD50) and 30 minutes later given Vitamin C (100mg/kg) [5]. The treatments were administered once daily by oral gavage for 2 weeks and all the rats were observed for clinical signs within the test period. In addition, the changes in the body weights of the rats in all the groups were measured before and after the experiment.
Evaluation of Hematological Parameters
Blood of rats from each group were collected directly from the heart after light ether anesthesia. Two milliliters of blood were collected into sample bottles containing ethylenediamine tetra acetic acid (EDTA). The hematological parameters that were evaluated included, total white blood cell count (WBC), total red blood cell count (RBC), mean cell volume (MCV), mean corpuscular hemoglobin concentration (MCHC), The evaluation was conducted with the use of an automatic hematological assay analyzer, Adva 60® hematology system (Bayer Diagnostics Europe Ltd., Ireland).
Histopathology
Brain samples were processed and stained using H and E as adopted by (6).
Statistical Analysis
Data generated during the experiment were expressed as mean ± SEM and subjected to one-way analysis of variance (ANOVA) followed by Tukey test using GraphPad Prism version 4.0 for windows from GraphPad Software, San Diego, California, USA (www.graphpad.com). Values of P less than 0.05 were considered significant.
Results
Clinical signs
The rats in group B showed clinical signs of salivation, restlessness, scratching and rubbing the mouth on beddings and diarrhea. The hair coat was rough and also there was erection. Rats in groups A and C did not exhibit any sign of toxicity while rats in Group D appeared dull.
Effect of Treatment on hematological parameters
Effect of treatments on white blood cell count (WBC)
There was no significant (p > 0.05) difference in the WBC counts of rats in groups A (15.1 × 103 µl ± 0.5), C (16.2× 103 µl ± 2.0) and D (14.4 × 103 µl ± 1.4). The lowest WBC count was recorded among rats in group B (11.74 × 103 µl ± 0.5) which was significantly (p less than 0.05) lower compared to those of groups A and C.
Effect of treatments on red blood cell count (RBC)
There was a significant (P less than 0.05) decrease in the RBC count of rats in group B (3.7 × 106 ul ± 0.5) as compared to those of groups A (7.4 × 106 ul ± 0.4), C (7.0 × 106 ul ± 0.8) and D (5.8 × 106 ul ± 0.2). No significant difference was observed in rats in groups A, C and D.
Effects of the treatments on Erythrocyte Indices
Effects of the treatments on Mean Cell Volume (MCV)
There was no significant (P > 0.10) difference in the MCV of rats in all groups. Group A (58.62(fl) ± 1.6), group B (57.18 (fl) ± 0.8) group C (59.82 (fl) ± 1.19) and group D (58.34 (fl) ± 1.0).
Effects of the treatments on Mean Cell Volume (MCHC)
There was significant (P less than 0.05) increase in the MCHC values of rats in group B (41.60g/dl ± 2.3) as compared to that of groups A (35.56g/dl ± 0.4), C (35.30 g/dl ± 0.63) and D (34.50g/dl ± 0.27). No significant (P > 0.05) difference was observed between groups A, C and D.
Table: Showing Effect of cypermethrin on mean body weight of Wistar rats.
| Weights (± SE) | ||
| Group | Initial (g) | Final (g) |
| A | 187(± 12.6) | 209 (± 21.5) * |
| B | 242 (± 15.5) | 243 (± 12.5) |
| C | 215 (± 13.2) | 235 (±13.6) * |
| D | 164 (±13.9) | 204 (±14.1) * |
N/B: Asterisk (*) shows groups that had significant weight changes
Histopatology
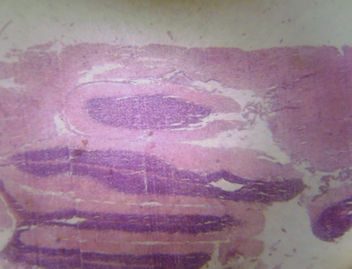
Figure 1: Photomicrograph of cerebrum of Rats treated with distilled water showing no apparent histopathological changes (H and E X100).
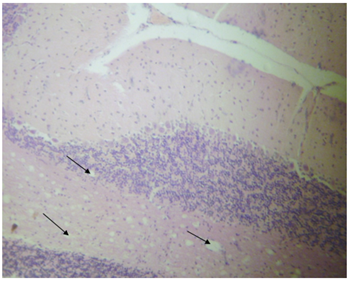
Figure 2: Photomicrograph of cerebrum of rats treated with cypermethrin showing vacuoles (arrows) (H and E X400).
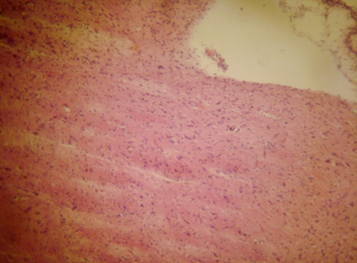
Figure 3: Photomicrograph of cerebrum rats treated with Vitamin C showing no apparent histopathological changes (H and E X400).
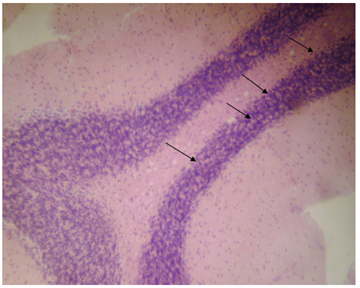
Figure 4: Photomicrograph of cerebrum rats treated with Cypermethrin and Vitamin C, showing vacuoles (arrows) (using H and E X100).
Discussion
The decreased WBC count seen in cypermethrin exposed rats may be due to either decreased production and/or increased rate of removal due to rapid destruction of WBC. Pesticides have been known to induce lymphopenia. In contrast, some researchers have documented an increase in WBC [7]. The increase in WBC may be indicative of activation of defense and immune system of the body following chronic exposure to cypermethrin. Treatment with vitamin C ameliorated the decreased WBC count noticed in the cypermethrin treated group. The reduction in RBC count may be due to hyperactivity of bone marrow [8], and consequently production of red blood cells with impaired integrity which can be destroyed easily in circulation by reticulo-endothelial system [9] suggests that the decrease in RBC counts is either indicative of excessive damage to erythrocytes or inhibition of erythrocytes formation caused by cypermethrin. The increased RBC count in group D showed that vitamin C protected the red cells from damage by prevention of free radicals’ formation [5]. Cypermethrin has been reported to evoke normocytic hyperchromic anemia in rats [10]. This explains why the erythrocytes in the group B exhibited normal sizes, with corresponding normal MCV. The normal value of MCV in rats is 50-65 (fl) [11]. The rats in group D had MCV values within the normal range showing that vitamin C protected the RBC from cypermethrin induced damage.
The increase in MCHC of group B agrees with the findings of [10] where cypermethrin caused a dose dependent increase in hemoglobin concentration. The current study shows that cypermethrin caused an increase in hemoglobin concentration with corresponding normal MCV values. The administration of cypermethrin in the present study elicited normocytic hyperchromic anemia in the Wistar rats. The findings from this study are consistent with results of [10] and [12] where cypermethrin and deltamethrin respectively did not cause significant body weight changes in rats. The increase in body weight changes seen in vitamin C treated group showed that vitamin C through its antioxidant mechanism. It is able to reduce damage caused by oxidizing chemicals, such as free radicals. Vitamin C reduces this damage by directly binding to oxidizing chemicals and converting them to less harmful molecules [5]. Cerebellum has two cell layers, the granular layers having more cells and the molecular layer having fewer cells. When they are responding to inflammation or injury from a toxicant produces vacuole which were seen in groups B and D as open spaces. Vacuoles results from swelling of cell processes after neuronal cell death. This was more in group B compared to those of group D.
Conclusion
Sub-acute exposure to cypermethrin induced hepatotoxic and brain histopathological changes Vitamin C ameliorated the hepatotoxic and brain histopathological changes following subacute exposure to cypermethrin. Declaration of generative AI and AI-assisted technologies in the writing process. During the preparation of this work the author(s) used Google/ Search engine in order to have an accurate reference. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Declarations
Funding Declaration
There was no funding for this research
Data Availability
Data is provided within the manuscript
Competing Interest Declaration
I declare that the authors have no competing interest as defined by BMC, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Ethical clearance
The committee on Animal use and care of the Usmanu Danfodiyo University, Sokoto, provided the ethical approval. The informed consent obtained for this research was written.
References
- Jerry, C and Hans D. (2007). The Benefits of Pesticides to mankind and the Environment. Cropro. 26(9):1337-1342.
Publisher | Google Scholor - Australia S. (2005). EPA Guidelines for Responsible Pesticide Use.
Publisher | Google Scholor - Ferrer A and Cabral R. (1995).
Publisher | Google Scholor - D Lorke. (1983). A new approach to practical acute toxicity testing. Archives of Toxicology, 54:275-287.
Publisher | Google Scholor - Ambali S, Akanbi D, Igbokwe N, Shittu M, Kawu M and Ayo J. (2007). Evaluation of subchronic chlorpyrifos poisoning on hematological and serum biochemical changes in mice and protective effect of vitamin C. The Journal of Toxicological Science, 32(2):111-120.
Publisher | Google Scholor - Bello A, Onyeanusi B. I, Sonfada M. L, Adeyanju J. B, Umar A. A, Umaru M. A, Shehu S. A and Hena S. A. (2012). Histomorphological Studies of the Prenatal Development of Oesophagus of One Humped Camel (Camelus dromedaries). Scientific Journal of Agricultural, 1(4):100-104.
Publisher | Google Scholor - Yousef M. I, Ibrahim H. Z, Yacuot M. H and Hassan A. A. (1998). Effects of cypermethrin and dimethoate on some physiological and biochemical parameters in Barki sheep. Egyptian Journal of Nutrition and Feeds, 1(1):41-52.
Publisher | Google Scholor - Tung H. T, Cook F. W, Wyatt R. D and Hamilton P. B. (1975). The anaemia caused by aflatoxin. Poultry Science, 54:1962-1969.
Publisher | Google Scholor - Shakoori A. R, Aziz F, Alam J and Ali S. S. (1990). Toxic effects of talstar, a new synthetic pyrethroid, on blood and liver of rabbit. Pakistan Journal of Zoology, 23:289-300.
Publisher | Google Scholor - Ferah S, Nafise U.K.Y, Yagit U, Huseyin A, Altug Y and Mehmet T. (2005). Neurotoxic effects of cypermethrin in wistar rats: A haematological biochemical and histopathological study. Journal Health Science, 51:300-307.
Publisher | Google Scholor - Gad S. C and Chengelis C. P. (1992). Animal models in Toxicology, Marcel Dekker inc. New York U.S.A, 884.
Publisher | Google Scholor - Aziz M. H, Agarawal A. K, Adhami V. M, Shukla Y And Seth P.K. (2001). Neuro developmental consequences of gestational exposure to low dose deltamethrin in rats. Neuroscience Letters, 300:161-165.
Publisher | Google Scholor