Research Article
Effect of Ethanol Administration on Cadmium and Magnesium Retention in Liver, Kidney and Heart of Rats
- Chidi J. Ogham *
- Jonathan D. Dabak
- Kiri. H. Jaryum
Department of Biochemistry, Faculty of Basic Medical Sciences, College of Health Sciences, University of Jos, Nigeria.
*Corresponding Author: Chidi J. Ogham, Department of Biochemistry, Faculty of Basic Medical Sciences, College of Health Sciences, University of Jos, Nigeria.
Citation: C J. Ogham, J D. Dabak, K. H. Jaryum (2023). Effect Of Ethanol Administration on Cadmium and Magnesium Retention in Liver, Kidney and Heart of Rats. Addiction Research and Behavioural Therapies, BRS Publishers. 2(1); DOI: 10.59657/2837-8032.brs.23.005
Copyright: © 2023 Chidi J. Ogham, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: March 02, 2023 | Accepted: March 29, 2023 | Published: April 11, 2023
Abstract
Background and objective(s): There were a health challenge in our study area with no known etiology, which affected mostly alcoholics. Water quality assessment in the area, indicated that it is contaminated with cadmium and had high magnesium content. The aim of this work, therefore, was to mimic the co-administration of cadmium and magnesium with graded concentrations of alcohol using a rat model to assess the retention of cadmium in the liver, kidney and heart, in order to give a plausible explanation on the cause(s) of this health challenge.
Methods: Rats were randomly divided into eight groups of 4 rats per group in metabolic cages. Group 1 served as normal control and fed with animal Feed and Water only. Group 2 was fed with animal feed and 6% Alcohol only (Test control). Group 3 to 8 were treated with the combination of cadmium, magnesium and graded concentrations of alcohol (aq). Treatment was carried out for a period of 21 days, with urine collection at the 7th, 14th and 21st day. The rats were sacrificed, tissues harvested and the assessment of the accumulation and excretion of these metals in tissues and in urine were performed by means of dry mineralization method and spectro-photometry.
Results: The results revealed that ethanol consumption increases the excretion of magnesium in the urine and increases cadmium retention in these tissues.
Conclusions: This suggest that alcoholics have higher risk of kidney, liver and heart injuries caused by cadmium toxicity in areas where the water source is polluted with cadmium as it is the case with our study area.
Keywords: cadmium; magnesium; alcohol; liver; kidney; heart
Introduction
Some years back, a health crisis of epic proportion was reported in Bar village, after the community source of water was changed from surface water to well water for drinking, cooking, and irrigation. The outbreak of the strange disease was characterized by migraine, fever, anaemia, hypertension, degenerative dementia and liver enlargement leading to the death of dozens of people in this village. The disease, according to the villagers started in October 2003. It was suspected to come from the consumption of the hand-dug well water in the area. Water quality assessment of the well water was carried out and the result indicated that it was contaminated with cadmium and had high magnesium content [1], and some toxic metals of hydro-geological origin. A local Daily, Trust newspaper, reported the occurrence of the incidence on Wednesday May 31, 2006 and the once lively villagers lived in fears of the diseases [2].
An epidemiology study carried out by the public health department of the Bauchi State Ministry of Health discovered that the deaths from these diseases were mainly drinkers of locally brewed alcoholic beverage produced in the area. The study was however rather suggestive because it could not show a clear link between the health effects, concurrent exposure of toxic metals and alcohol consumption.
Environmental pollution and contamination by heavy metals is a threat to the ecosystem, and is of serious health concern to humans in this modern age, mainly due to growth in industrialization and urbanization [3]. The mobilization and transportation rates of these metals in the environment have greatly increased since the 1940s [4]. These metals occur naturally in the environment through weathering of metal-containing rocks and volcanic eruptions, while industrial emissions, mining, smelting, and agricultural activities like application of pesticides and phosphate fertilizers are principal anthropogenic sources5. Heavy metals contaminate the food chains, persist in the environment, and cause different health problems due to their toxicities.
It should be emphasized that exposure to these toxic metals most often involves exposure to a combination of toxic metals rather than to just a single metal; thus, accurate assessment of health risk due to drinking metal-contaminated groundwater must consider potential interactions between the metals on the chemical, biochemical, and physiological levels. When subjects are exposed to metals in combination, the metals may each have their own typical health effects; either synergistically or antagonistically. In estimating the risks due to drinking water contaminants, it is vital to consider not only the health risks due to individual contaminants, but also those risks due to the combinations of contaminants. Ingestion of metals in combination may increase or decrease the absorption and distribution of the individual metals in the digestive tract and circulatory system as well as also affecting the excretion of other metals. [6,7] Absorption of metals through drinking water could be affected by certain diets, behaviours and addiction. Interactions with dietary components and the general nutritional levels also affect the outcomes of exposure to toxic metals [8]. Behavioral and cultural practices (e.g., smoking) and dietary habits (alcoholism) also affect health due to co-exposures to toxic metals. Particularly interesting are interactions between xenobiotics to which exposure is not often common. Examples of such substances are cadmium and ethanol. Interactions between cadmium and ethanol is an important problem in the field of modern toxicology, as both substances pose a risk to human and animal health [9]. Dabak et al. (2016) also reported that magnesium possesses some level of protective effect against cadmium toxicity [10].
There are reports implicating ethanol in increasing the permeability of biological membranes to cadmium [11], which can make alcoholics more susceptible to the effect of cadmium toxicity [12]. Liver and kidney are the important organs of metabolism, detoxification, storage and excretion of xenobiotics and their metabolites, and are especially vulnerable to damage. As the liver is an important target organ of ethanol [13], and the kidney of Cd toxicity [12,14]. Particulate matter exposures result in the delivery of metals to multiple extra-pulmonary sites of the heart where they form reactive centers that continually catalyze the generation of reactive oxygen species and induce oxidative stress. The design of this work was therefore conceived to investigate the possible effects of the concurrent administration of cadmium and magnesium with graded concentrations of alcohol on the distribution, accumulation and retention of these metals on the liver, kidney and heart as well as the urine concentrations. This was to enable us establish the etiology of the cause of deaths mostly by alcoholics in our study area using a rat model to estimating the interactions between parameters in subject to support relevant in vitro data, findings and correlations.
Material and Methods
Study Design
Rats were randomly divided into eight groups of 4 rats per group in cages and all the groups drank the solution meant for each group ad libitum. The administration of the ethanol at a dose of 5 g of 96% ethanol/kg body wt/24 h was done through intragastric intubation at intervals of 12 hours all through the period of the experiment. During the whole course of the experiment, the animals were kept under identical conditions and had unlimited access to feed. As a case study for events taking place in Bar/Bula village, we have used cadmium and magnesium exposure level to correspond to those that occur in the ground water sampled in the area. Preparations of aqueous solutions containing 0.16mg/L of CdCl2 and 105mg/L of MgCl2 were served as representative water samples administered to the rats.
Group 1 (Normal control) was placed on redistilled water to drink for the whole course of the experiment. Group 2 (Test control) was treated with 6% (v/v) aqueous solution of ethanol only. Group 3 was treated with 1% (v/v) aqueous solution of ethanol and drinking aqueous solution of the combination of 0.16mg/L of CdCl2 and 105mg/L of MgCl; Group 4 treated with 2% (v/v) aqueous solution of ethanol and drinking aqueous solution of 0.16mg/L of CdCl2 and 105mg/L of MgCl; Group 5 was treated with 3% (v/v) aqueous solution of ethanol and drinkingaqueous solution of 0.16mg/L of CdCl2 and 105mg/L of MgCl; Group 6 was treated with 4% (v/v) aqueous solution of ethanol and drinkingaqueous solution of 0.16mg/L of CdCl2 and 105mg/L of MgCl; Group 7 was treated with received 5% (v/v) aqueous solution of ethanol and drinkingaqueous solution of 0.16mg/L of CdCl2 and 105mg/L of MgCl; Group 8 was treated with 6% (v/v) aqueous solution of ethanol and drinkingaqueous solution of 0.16mg/L of CdCl2 and 105mg/L of MgCl. The animals were included in the study if they underwent successful intragastric intubation throughout the study period. The animals were excluded if they died prematurely before collection of analytical specimens.
During the course of the study, four different teams of investigators were involved; a first investigator was responsible for treatments preparation. A second investigator was responsible for administration of the treatment based on the groupings. A third investigator was responsible for anaesthetic procedure and performance of the surgical procedure whereas a fourth group of investigators (unaware of treatment) assessed the biochemical and histopathological examinations.
Experimental Animals
Thirty-two (32) 10-week-old white albino rats with average body weights of 230g were obtained from the animal house of the University of Jos, Nigeria and were fed commercial feed (Vital Feed) and provided with various treatment water ad libitum. They were housed in ventilated cages and maintained on a regular diurnal lighting cycle (12:12 light: dark). Chopped corn cob was used in bedding. They were allowed to acclimatize for 7 days under standard environmental conditions before treatments.
Ethical Clearance
All experimental procedures were approved by the Ethical Committee on Animal Experiment unit of the University of Jos, Faculty of Pharmaceutical Sciences Reference number: F17-00379, in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996).
Chemicals/Reagents
The feed used was vital feed (growers mash) produced by Grand Cereal and Oil Mills Ltd, Jos Plateau state. The cadmium chloride used was a product of May and Baker (M&B) Ltd, Gagenhan, England while ethanol was a product of British Drug House (BDH), England. Magnesium chloride used was a product of Sigma-Aldrich. Other chemicals used were of analytical grade purchased by Ministry of Education, Bauchi state for Government Science Secondary School Misau and from NanaRich Medical Laboratories Bauchi and Prestige Laboratories Jos. All chemical preparations were done with distilled water, which was distilled from pyrex apparatus. Standards of Cadmium were obtained from Water and Sanitation Agency (WATSAN) laboratory established by United Nations Children Fund (UNICEF) at Bauchi.
Sample Collection and Preparation
Urine samples from rats were collected after every seven (7) days interval with sterile leak proof containers from the urine collector of the metabolic cages, after which the urine samples were kept frozen until needed for clinical analyses. After 21 days’ administration, the rats were sacrificed by cervical dislocation. The kidney, heart and liver were harvested and stored at -20oC for tissue concentration of metals.
Determination of Concentrations of cadmium and magnesium in tissues and urine samples
Samples of known weight(slices) were subjected to dry mineralization in an electricoven according to Zmudzki (1977) [15]. The ash was dissolved in a known volume of 1N HCl (bone) or HNO3 (soft tissues). The concentrations of all metals in such preparations and urine (after appropriate dilution) were assessed by atomic absorption spectrophotometry (Zeiss Jena, Germany AAS 30) with electrothermal atomization in a graphite cuvette (cadmium) or flame atomization in an air–acetylene burner (magnesium). The cathode lamps of the respective elements were operated under standard conditions using their respective resonance lines: Cd, 228.8 nm and Mg, 285.2 nm. The concentrations of metals were expressed as g or mg/g of fresh tissue, g/24-h urine collection.
Results
Tissue Metal Concentration
Cadmium Tissue Concentration
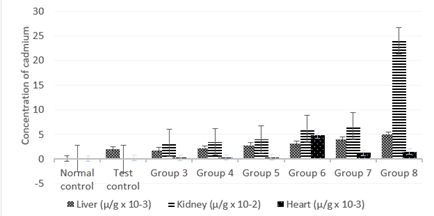
Figure 1: Effect of the co-administration of cadmium, magnesium and alcohol on cadmium deposits levels of liver, kidney and heart.
Figure 1 shows significantly high (P less than 0.05) concentration of cadmium levels in the liver for all groups when compared to the control. Increase in the concentration of alcohol caused a progressive and significant (P less than 0.05) increase in cadmium deposit levels in the liver from group 3 to 8 of the rats’ treatment. The results also show significantly high (P less than 0.05) values of cadmium levels in the kidney for all groups when compared to the control. Increase in the concentration of alcohol caused a progressive and highly significant (P less than 0.05) increase in cadmium deposit levels in the kidney from group 3 to 8 of the rats’ treatment with group 8 standing out. In addition, there was significant increase (P less than 0.05) concentration of cadmium levels in the heart for all groups when compared to the control. Increase in the concentration of alcohol caused a progressive and significant (P less than 0.05) increase in cadmium deposition in the heart from group 3 to 6 and the subsequent decline in groups 7 and 8.
Magnesium Tissue Concentration
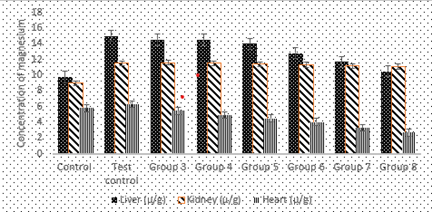
Figure 2: Effect of the co-administration of cadmium, magnesium and alcohol on magnesium concentration levels in rat liver, kidney and heart
Figure 2 shows significantly high (P less than 0.05) values of magnesium levels deposits in the liver for all groups when compared to the control. Increase in the concentration of alcohol caused a progressive and significant decrease in magnesium deposit levels in the liver from group 3 to 8 rats.
There was also significantly high (P less than 0.05) concentration of magnesium levels deposited in the kidney for all groups when compared to the control. Increase in the concentration of alcohol caused a progressive but marginal decrease in magnesium deposition levels in the kidney from group 3 to 8 the rats. There were significantly high (P less than 0.05) values of magnesium levels deposits in the heart in group 2 when compared to the control but shows significantly low (P less than 0.05) values of magnesium levels deposited in the heart for groups 3 to 8 when compared to the normal control. Increase in the concentration of alcohol caused a progressive and significant (P less than 0.05) decrease in magnesium deposit levels in the heart from group 3 to 8 rats.
Urine Metal Excretion
Cadmium Urine Concentration
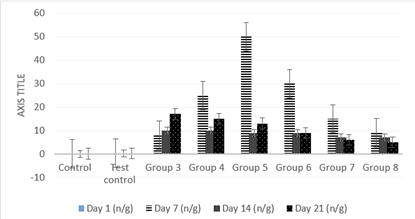
Figure 3: Effect of co-administration of cadmium, magnesium and alcohol on urine cadmium concentration levels on day 1, 7, 14 and 21.
Figure 3 shows significantly low (P less than 0.05) values in cadmium urine excretion levels on the first day of treatments for all groups when compared to the control. There were also significantly high (P less than 0.05) values in cadmium urine excretion levels after the first 7 days of treatments for all groups when compared to the control. Increase in the concentration of alcohol caused a progressive and highly significant (P less than 0.05) increase in cadmium urine excretion levels after the first week from group 3 to 5 of the rats’ treatment with subsequent progressive decline from groups 6 to 8. Results also show that there were significantly high (P less than 0.05) values in cadmium urine excretion levels after the second 7 days of treatments for all groups when compared to the control. Increase in the concentration of alcohol caused a progressive and significant (P less than 0.05) decrease in cadmium urine excretion levels after the second week from group 3 to 8 of the rats’ treatment. There were significantly high (P less than 0.05) values in cadmium urine excretion levels after the third 7 days of treatments for all groups when compared to the control. Increase in the concentration of alcohol caused a progressive and highly significant (P less than 0.05) decrease in cadmium urine excretion levels after the third week from group 3 to 8 of the rats’ treatment.
Magnesium Urine Concentration
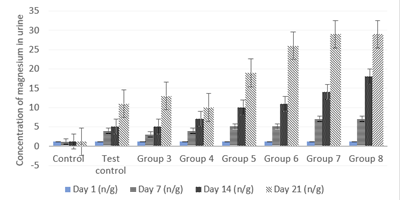
Figure 4: Effect of the co-administration of cadmium, magnesium and alcohol on urine magnesium excretion levels on day 1, 7, 14 and 21.
Figure 4 shows values equal to Tissue Metal ConcentrationFigure 4 shows values equal to (P less than 0.05) values in magnesium urine excretion levels on the first day of treatments for all groups when compared to the control. There were significantly high (P less than 0.05) values in magnesium urine excretion levels after the first 7 days of treatments for all groups when compared to the control. Increase in the concentration of alcohol caused a progressive and highly significant (P less than 0.05) increase in magnesium urine excretion levels after the first week from group 3 to 8 of the rats’ treatments.
There were also significantly high (P less than 0.05) values in magnesium urine excretion levels after the second 7 days of treatments for all groups when compared to the control. Increase in the concentration of alcohol caused a progressive and highly significant (P less than 0.05) increase in magnesium urine excretion levels after the second week from groups 3 to 8 treatments. Results also show significantly high (P less than 0.05) values in magnesium urine excretion levels after the third 7 days of treatments for all groups when compared to the control. Increase in the concentration of alcohol caused a progressive and highly significant (P less than 0.05) increase in magnesium urine excretion levels after the third week from group 5 to 8 treatments.
values in magnesium urine excretion levels on the first day of treatments for all groups when compared to the control. There were significantly high (P less than 0.05) values in magnesium urine excretion levels after the first 7 days of treatments for all groups when compared to the control. Increase in the concentration of alcohol caused a progressive and highly significant (P less than 0.05) increase in magnesium urine excretion levels after the first week from group 3 to 8 of the rats’ treatments.
There were also significantly high (P less than 0.05) values in magnesium urine excretion levels after the second 7 days of treatments for all groups when compared to the control. Increase in the concentration of alcohol caused a progressive and highly significant (P less than 0.05) increase in magnesium urine excretion levels after the second week from groups 3 to 8 treatments. Results also show significantly high (P less than 0.05) values in magnesium urine excretion levels after the third 7 days of treatments for all groups when compared to the control. Increase in the concentration of alcohol caused a progressive and highly significant (P less than 0.05) increase in magnesium urine excretion levels after the third week from group 5 to 8 treatments.
Discussion
Effects on Tissue Concentration of Metal
Cadmium accumulation and excretion in urine
Our results had shown that there was significantly high (P<0>
As a non-essential element, it is unlikely that Cd enters the body via a Cd-specific transport mechanism but rather by crossing various membranes utilizing the transport mechanism of other divalent metal ions, including Ca/Mg transporter [25, 26]. As is evident from the study, cadmium uptake increases significantly when given to rats in conjunction with alcohol. Studies have indicated that alcohol can induce in vivo changes in membrane lipid composition and fluidity [27], which may affect cellular functions. Alcohol induces membranes fluidity, thereby making them more permeable to cadmium [28], which will invariably make alcoholics more susceptible to the hazards of cadmium as compared to their non-alcoholic counterparts even in the absence of direct occupational exposure. The increase in the uptake of cadmium from the drinking water could be due to the increased permeability of membrane in the presence of ethanol. The observations made in this study indicate that ethanol administered at high concentration even for a short period of time with a simultaneous exposure to cadmium increase the concentration of this heavy metal. A decrease in cadmium urinary excretion in rats exposed to ethanol and the increased excretion of magnesium suggests that ethanol increases cadmium retention in tissues. When these two metals are present in the cell concurrently, they are mutually exclusive depending on their concentrations.
Magnesium accumulation and excretion in urine
Absorption of magnesium in the body is mostly via the small intestine by a passive paracellular mechanism, which is driven by an electrochemical gradient and solvent drag [29]. A minor, but important, regulatory fraction of magnesium is transported through the transcellular transporter transient receptor potential channel melastat in member (TRPM) 6 and TRPM7 - members of the long transient receptor potential channel family - which also play an important role in intestinal calcium absorption [30]. Proven pathologic mechanisms have revealed that cadmium exhibit competitive and inhibitory interference with the physiologic uptake of Mg [31, 32]. However, habitually low intakes or excessive losses of magnesium due to chronic alcoholism can lead to magnesium deficiency [33] and invariably leading to cadmium accumulation and deposition when they administered together. Other than causing these direct effects on various organs of rats, cadmium also affects essential trace metal homeostasis. As can be seen from the study, a significant decrease in magnesium concentration was observed in Cd-Mg/Et treated rats in all the tissues. This decrease is progressive as the concentration of alcohol increases. Administration of ethanol and in conjunction with cadmium/magnesium decreased magnesium concentration in the liver and kidney with increase concentration the ethanol. Magnesium is known to serve a protective role when present in the cell with toxic metals such as cadmium [34].
It has been reported that the heart does not exhibit any metallothionein-inducing capacity [35]. This observation is relevant since it suggests that the heart may be more prone to cadmium toxicity because it lacks metallothionein that binds to and detoxifies Cd. Our data show a progressive decrease in Mg levels in liver, kidney and heart of the treated rats as a result of increased excretion of magnesium due to the presence of alcohol. The result of this work agrees with previous works which reported that chronic alcoholics have altered Mg homeostasis [33,36]. Marked hypomagnesemia associated with hypomagnesuria has also been observed in alcoholics.33 From our data, a significant decrease in the magnesium levels was observed in the liver of Cd/Mg and ethanol co-exposed rats relative to controls although this decrease was not as pronounced as in rats treated with cadmium/magnesium alone. Further, a significant decrease (p less than 0.05) in magnesium concentration as compared to controls was observed in kidney and heart in the Cd/Mg and ethanol co-exposed group. This could be due to inadequate synthesis of metallothionein in response to the increased concentration of Cd in these organs of the Cd and ethanol co-exposed groups. The hypomagnesuria associated with ethanol consumption might further decrease the levels of magnesium in this particular group. Thus, any change in the homeostasis of these essential trace metals can also be detrimental to the activity of these enzymes.
Excessive Cd in the liver also depresses hepatic functions and results in decreased synthesis of metalloenzymes [37]. Ethanol, both as a protein synthesis initiator and as an acute potentiator of Cd up take, aggravates the situation. The mechanism of the ethanol-induced increase in cadmium accumulation in soft tissues may be due to the ability of both substances to induce metallothionein synthesis [33]. Ethanol had been implicated in the induction of metallothionein synthesis indirectly by increasing the cadmium body burden, as well as by an alteration in zinc and glucocorticoid homeostasis [38]. An excessive exposure to cadmium and its accumulation in the organism lead to disturbances in metabolism of essential elements [39].
The interactions between cadmium and magnesium can take place at different stages of their metabolism: absorption from the gastrointestinal tract, distribution in the organism and excretion in urine, as well as at the stage of biological functions of essential elements [28]. This increased level of Cd further perturbs the homeostasis of other essential trace metals such as zinc and copper, which may eventually disturb the synthesis and functioning of vital metalloenzymes [28].
Administration of ethanol alone caused an increase in magnesium concentration in the tissues. In this study, the water intake of rats and the urine output were not taken into consideration; however, we can speculate that addition of cadmium into drinking water resulted in a significant decrease in the water consumption. Solutions containing Cd as well as ethanol have a bad taste and thus animals develop aversion to drinking them [40]. This will however, lead to the reduction of the urine output. This also serve as the basis why alcohol was intragistically administered during the treatments.
Summary of Findings
From the study, findings revealed that ethanol consumption increases the excretion of magnesium in the urine and increases cadmium uptake and accumulation in the liver, kidney and the heart.
Conclusion
The results of this study revealed that ethanol consumption increases the excretion of Mg in the urine and increases Cd uptake in the liver, kidney and the heart. This means that alcoholics have higher risk of kidney, liver and heart Cd toxicities in areas where the water source is polluted with cadmium, as is the case with our study area.
Declarations
Authors’ Contribution
CO conceived, researched and funded the study; CO also drafted the manuscript; JD supervised and provided significant input to several sections to improve clarity and accuracy; KJ reviewed, edited validated the draft. All authors read and approved the final manuscript.
Acknowledgements
The authors posthumously acknowledge the support of late Engr. A. Marafa, a former Director Bauchi State ministry of water resources and rural development, at this stage of the study. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict Of Interests
The authors declare that there are no conflicts of interest related to this study.
Funding Source
This work was self-funded.
References
- Ogham CJ, Dabak JD and Jaryum KH. (2021). Physicochemical and metal assessment of Bar village well water and the effect of co-administration of cadmium, magnesium and alcohol on some organs. Unpublished PhD Thesis, University of Jos, Plateau State, Nigeria, 148-149.
Publisher | Google Scholor - Tashikalmah H. (2006). Bauchi: Villagers raise alarm over waterborne disease. Daily Trust; May 31, p.5.
Publisher | Google Scholor - Hashem, MA, Nur-A-Tomal, Mondal, & Rahman, MA. (2017). Hair burning and liming in tanneries is a source of pollution by arsenic, lead, zinc, manganese and iron. Environmental Chemistry Letters, 15(3), 501-506.
Publisher | Google Scholor - Khan FU, Rahman AU, Jan A & Riaz M. (2004). Toxic and trace metals (Pb, Cd, Zn, Cu, Mn, Ni, Co and Cr) in
Publisher | Google Scholor - Alengebawy A, Abdelkhalek ST, Qureshi SR, Wang MQ. (2021). Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics. 9(3):42.
Publisher | Google Scholor - Ahmed F, & Ishiga H. (2006). Trace metal concentrations in street dusts of Dhaka city, Bangladesh. Atmospheric Environment, 40(21), 3835-3844.
Publisher | Google Scholor - Dabak JD, Gazuwa SY and Ubom GA. (2018). Effect of grade concentrations of Ca on nephrotic cells of Cd and Pb co-intoxicated rats. Journal of Environmental Toxicology and public Health 3: 9-17.
Publisher | Google Scholor - Koboldt DC, Fulton RF, McLellan M, Schmidt H, et.al. (2012). Comprehensive molecular portraits of human breast tumours. Nature, 490(7418), 61-70.
Publisher | Google Scholor - Omar AMS. (2013). Histopathological and physiological effects of liver and kidney in rats exposed to cadmium and ethanol. Global Advanced Research Journal of Environmental, 2(3), 93-106
Publisher | Google Scholor - Dabak JD, Gazuwa SY and Ubom GA. (2017). Nephroprotective effect of graded concentrations of Mg on cd and Pb co-intoxicated rats. Journal of Basic and Applied Research International. 23(1):41-50.
Publisher | Google Scholor - Brzóska MM, Galażyn‐Sidorczuk M & Dzwilewska I. (2013). Ethanol consumption modifies the body
Publisher | Google Scholor - Prozialeck WC & Edwards JR. (2012). Mechanisms of cadmium-induced proximal tubule injury: new insights with implications for biomonitoring and therapeutic interventions. Journal of Pharmacology and Experimental Therapeutics, 343(1), 2-12.
Publisher | Google Scholor - Teschke R. (2018). Alcoholic Liver Disease: Alcohol Metabolism, Cascade of Molecular Mechanisms, Cellular Targets, and Clinical Aspects. Biomedicines. 12; 6(4):106.
Publisher | Google Scholor - Nordberg GF, Jin T, Nordberg M. (1994). Subcellular targets of cadmium nephrotoxicity: cadmium binding to renal membrane proteins in animals with or without protective metallothionein synthesis. Environ Health Perspect. 102 Suppl 3(Suppl 3):191-4.
Publisher | Google Scholor - Zmudzki J. (1977). “Oznaczanie zawartosci olowiu w material biologicznym metoda spektrofotometrii
Publisher | Google Scholor - Dabak JD, Gazuwa SY. and Ubom GA. (2011). Comparative hepatotoxicity test of cadmium and lead in
Publisher | Google Scholor - Brzóska MM, Moniuszko-Jakoniuk J, Piłat-Marcinkiewicz B, Sawicki B. (2003). Liver and kidney function and histology in rats exposed to cadmium and ethanol. Alcohol and Alcoholism 38(1):2-10.
Publisher | Google Scholor - Sabolić I, Breljak D, Skarica M, Herak-Kramberger CM. (2010). Role of metallothionein in cadmium
Publisher | Google Scholor - Satarug S, Vesey DA & Gobe GC. (2017). Health risk assessment of dietary cadmium intake: do current guidelines indicate how much is safe? Environmental health perspectives, 125(3), 284-288.
Publisher | Google Scholor - Hyder O, Chung M, Cosgrove D, Herman JM, et, al. (2013). Cadmium exposure and liver disease among US adults. Journal of Gastrointestinal Surgery, 17(7), 1265-1273.
Publisher | Google Scholor - Maret W, Moulis JM. (2013). The bioinorganic chemistry of cadmium in the context of its toxicity. Met
Publisher | Google Scholor - Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A. (2020). The Effects of Cadmium Toxicity. Int
Publisher | Google Scholor - Messner B, Knoflach M, Seubert A, Ritsch A, et, al. (2009). Cadmium is a novel and independent risk
Publisher | Google Scholor - De Silva, DD, Rapior S, Hyde KD & Bahkali AH. (2012). Medicinal mushrooms in prevention and control of diabetes mellitus. Fungal Diversity, 56(1), 1-29.
Publisher | Google Scholor - Roggeman S, de Boeck G, De Cock H, Blust R, Bervoets L. (2014). Accumulation and detoxification of metals and arsenic in tissues of cattle (Bos taurus), and the risks for human consumption.
Publisher | Google Scholor - Martelli A, Rousselet E, Dycke C, Bouron A, Moulis JM. (2006). Cadmium toxicity in animal cells by interference with essential metals. Biochimie. 88(11):1807-14.
Publisher | Google Scholor - Luo J, Ye Y, Gao Z, & Wang W. (2014). Essential and nonessential elements in the red-crowned crane Grus japonensis of Zhalong Wetland, northeastern China. Toxicological & Environmental Chemistry, 96(7), 1096-1105.
Publisher | Google Scholor - Winiarska-Mieczan A. (2013). Protective effect of tannic acid on the brain of adult rats exposed to cadmium and lead. Environ Toxicol Pharmacol. 36(1):9-18.
Publisher | Google Scholor - de Baaij JH, Hoenderop JG, Bindels RJ. (2012). Regulation of magnesium balance: lessons learned from
Publisher | Google Scholor - Volpe SL Magnesium. In: Erdman JW, Macdonald IA, Zeisel SH, eds. (2012). Present Knowledge in Nutrition. 10th ed. Ames, Iowa; John Wiley & Sons: 459-74.
Publisher | Google Scholor - Whittaker MH, Wang G, Chen XQ, Lipsky, et,al. (2011). Exposure to Pb, Cd, and As mixtures potentiates the production of oxidative stress precursors: 30-day, 90-
Publisher | Google Scholor - Wang L, Gallagher EP. (2013). Role of Nrf2 antioxidant defense in mitigating cadmium-induced oxidative stress in the olfactory system of zebrafish. Toxicol Appl Pharmacol. 266(2):177-86.
Publisher | Google Scholor - Ross AC, Caballero BH, Cousins RJ, Tucker KL, Ziegler TR. (2012). Modern nutrition in health and disease: Eleventh edition. Wolters Kluwer Health Adis (ESP). 1616 p.
Publisher | Google Scholor - Dabak JD, Gazuwa SY and Ubom GA. (2015). The Nephroprotective Effects of Graded Concentrations of Calcium and Magnesium on Nephrotoxicities Induced by a Constant Toxic Concentration of Cadmium and Lead in Rats. International Journal of Biochemistry Research and Review. 7(1): 36-44.
Publisher | Google Scholor - Ravi K. (1986). Biochemical Studies on Iso-metallothioneins in Monkeys Exposed to Cadmium, Ph.D. dissertation, Post graduate Institute of Medical Education and Research, Chandigarh, India.
Publisher | Google Scholor - McClain CJ, Su LC. (1983). Zinc deficiency in the alcoholic: a review. Alcohol Clin Exp Res. Winter 7(1):5-10.
Publisher | Google Scholor - Amamou F, Nemmiche S, Meziane RK, Didi A, Yazit SM, et, al. (1983).Protective effect of
Publisher | Google Scholor - Klaassen CD, Liu J, Diwan BA. (2009). Metallothionein protection of cadmium toxicity. Toxicol Appl Pharmacol. 238(3):215-20.
Publisher | Google Scholor - Brzóska MM, Galażyn‐Sidorczuk M & Dzwilewska I. (2013). Ethanol consumption modifies the body turnover of cadmium: a study in a rat model of human exposure. Journal of Applied Toxicology, 33(8), 784-798.
Publisher | Google Scholor - Mohamed TM, Salama AF, Nimr TME & Gamal DME. (2015). Effects of phytate on thyroid gland of rats
Publisher | Google Scholor