Case Report
Drug Interaction between Levetiracetam and Apixaban in A Young Patient with Ischemic Stroke
1Professor and Head, Department of Cardiology, AJIMS&RC, Mangalore, India.
2Consultant, Department of Neurology, AJIMS&RC, Mangalore, India.
3Senior Resident, Department of Cardiology, AJIMS&RC, Mangalore, India.
*Corresponding Author: Manjunath Bagur, Professor and Head, Department of Cardiology, AJIMS&RC, Mangalore, India.
Citation: Bagur M, Rai S, Shivakumar N, Ummar J. (2024). Drug Interaction between Levetiracetam and Apixaban in A Young Patient with Ischemic Stroke, Journal of Clinical Cardiology and Cardiology Research, BioRes Scientia Publishers. 3(1):1-5. DOI: 10.59657/2837-4673.brs.24.024
Copyright: © 2024 Manjunath Bagur, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: February 06, 2024 | Accepted: March 04, 2024 | Published: March 12, 2024
Abstract
Background: Newer oral anticoagulants (NOACs) are commonly used in patients with atrial fibrillation & venous thromboembolism and cardioembolic stroke. However, NOACs have important potential drug-drug interactions (DDIs) with several classes of drugs, particularly with antiepileptic (AE) drugs which may induce cytochrome P4503A4 or P-glycoprotein(P-gp). Co-administration of NOACs and AE drugs may result in lower NOAC drug levels and a reduction in its efficacy. Recent guidelines from the American Society of Hematology suggest that clinicians avoid prescribing P-glycoprotein inducers or inhibitors for patients who are taking NOACs [1]. The reason is that P-glycoprotein critically regulates the absorption, distribution, and secretion of NOACs [2]. Animal studies suggest that levetiracetam, a commonly used medication to prevent seizures, acts as a P-glycoprotein inducer to reduce NOAC plasma levels [2,3]. However, not everyone is convinced that levetiracetam should be avoided in patients receiving rivaroxaban because there is little or no published evidence describing this interaction in humans [4].
Objectives: Presenting a case report of levetiracetam affecting Apixaban efficacy of a young patient with ischemic stroke.
Conclusion: The current literature assessing these adverse clinical outcomes from NOACs and AE drug co-administration are limited. Although the available data point to a possible increased risk of thrombotic events, they are insufficient to conclude. Well-designed clinical studies are needed to arrive at definite conclusions.
Keywords: apixaban; levetiracetam; atrial fibrillation; ischemic stroke; drug interactions
Introduction
NOACs such as dabigatran, rivaroxaban, apixaban, and edoxaban, have emerged as the first-choice medication for treating and preventing venous thromboembolism (VTE) and preventing stroke in patients with atrial fibrillation (AF) throughout the last ten years. Drug-drug interactions (DDIs) with NOACs are less common than with vitamin K antagonists; yet, there are still some concerns about potential clinically significant NOAC DDIs. NOAC absorption is mediated by P-gp proteins, and, therefore, P-gp inhibitors potentially increase NOAC absorption and effect, whereas P-gp inducers potentially decrease NOAC absorption and effect. Furthermore, CYP3A4 enzymes are involved in the metabolism of oral direct Xa inhibitors, but not dabigatran and strong inhibitors of CYP3A4 enzymes potentially decrease the metabolism and increase direct Xa inhibitor effect, whereas inducers of CYP3A4 enzymes potentially increase the metabolism and decrease direct Xa inhibitor effect. It is uncertain whether patients who require coadministration of potentially interacting drugs and NOACs would have better outcomes if instead of a NOAC they received another anticoagulant. We found no systematic reviews that addressed this question [1,2]. There were no randomized trials that addressed the outcomes prioritized for this question.
The European Heart Rhythm Association advised against using NOACs in combination with carbamazepine, phenytoin, or phenobarbital until 2018 because those antiepileptic medications strongly increase CYP3A47 and P-gp. Levetiracetam was added to the list of antiepileptic medications that should not be taken with NOACs in 2018 when the guidelines were revised. Since there was insufficient proof that levetiracetam would cause P-gp or CYP3A4-mediated pharmacological interactions with NOACs, this caused great concern [5,6]. Levetiracetam is also a commonly used medication that effectively manages seizures. Its lack of protein binding and hepatic metabolism means that it shouldn't interact with other medications in a way that would be clinically significant. It also enhances quality of life and is well tolerated [7,8].
Case report
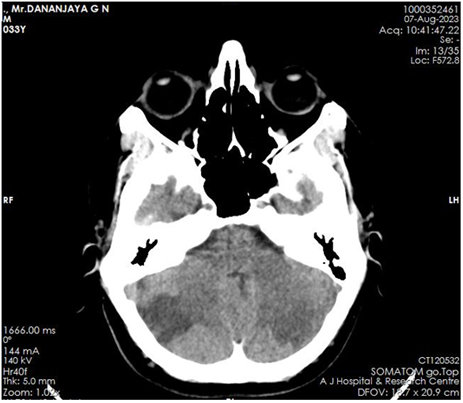
A 33-year-old male, with no known co-morbidities presented with young onset ischemic stroke involving left posterior cerebral artery territory and bilateral cerebellar region. Brain computed tomography (CT) [Figure 1] and Magnetic resonance imaging (MRI) demonstrated acute infarcts in the bilateral cerebellar region and left occipital lobe, the anteromedial surface of the temporal lobe, midbrain, and thalamus.
Figure 1: acute infarcts in left thalamus, subcortical white matter of left occipital lobe and bilateral cerebellar hemispheres. No areas of hemorrhagic transformation.
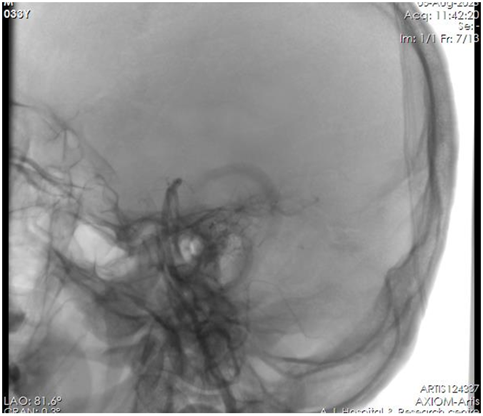
The patient was subjected to Cerebral digital subtraction angiography (DSA) [Figure 2] and there was no evidence of stenosis or atherosclerotic changes in vertebral and basilar arteries. Normal Sinus Rhythm was seen on the ECG. Transthoracic and transesophageal echocardiography revealed normal chambers and valves, no regional wall motion abnormalities, good bi-ventricular function, and no evidence of thrombus/vegetations with intact Inter atrial and ventricular septum. 72-hour Holter recording revealed no arrhythmic episodes. Serum homocysteine levels, vasculitis, and the thrombophilia profile were all within normal ranges. Cardioembolic stroke was more likely because the infarcts were noted in the left Posterior communicating artery/fetal Posterior cerebral artery area and the posterior cerebellum (bilateral cerebellar hemisphere). He received apixaban at a dose of 5 mg twice daily along with daily aspirin at a dose of 75mg and levetiracetam at 500mg twice daily and other guideline-directed medical therapy.
Figure 2: No Stenosis or Atherosclerotic Changes.
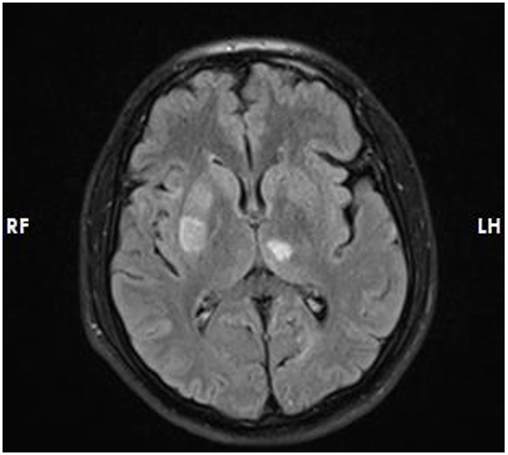
Nevertheless, the patient returned after 30 days with recurrent ischemic stroke and there were no areas of hemorrhagic alteration visible on a brain CT scan, The brain MRI [Figure 3] revealed acute infarcts in the right basal ganglia, anterior limb of the internal capsule, and pons with acute lacunar infarcts in left cerebellar hemisphere. To find out if low apixaban levels could account for the recurrent ischemic events, we examined his laboratory measurement of apixaban using anti-factor Xa assays (Anti-Xa assay; Instrumentation Laboratory) and found it to be in the sub-therapeutic range. He had been taking levetiracetam at the same dosage at that point. We accepted his declaration and his family’s confirmation of his strict adherence to apixaban and aspirin. As a result, we consulted with a neurologist discussed possible drug interaction, and considered switching apixaban with Vitamin K epoxide reductase complex 1 (VKORC1) inhibitor Warfarin. After starting Warfarin daily and other medications by guidelines and closely monitored to-date follow-up, there hasn't been another ischemic stroke episode.
Figure 3: Acute Infarcts in Right Basal Ganglia, Anterior Limn of Internal Capsule and Pons and Acute Lacunar Infarcts in Left Cerebellar Hemisphere.
Discussion
Levetiracetam is one of the first-line antiepileptic drugs due to its favorable pharmacokinetic profile, efficacy, and tolerability [8]. It does not induce CYP 3A4 activity [9,10], but it was found to be a substrate for human P-gp in in-vitro cell line models [11]. Induction of P-gp by levetiracetam was demonstrated in mice models [12]. However, in-vitro data suggests species-specific recognition by P-gp. It was shown that levetiracetam was transported by mouse, but not human P-gp [13]. Studies on healthy volunteers receiving levetiracetam and the P-gp substrate digoxin, as well as recent in-vitro studies on the expression and function of ATP binding cassette transporters, did not confirm levetiracetam as a P-gp inducer [14,15]. However, we should keep in mind that animal models and in-vitro systems cannot thoroughly mimic the complexity of human clinical conditions. No systematic studies investigating NOAC levels in patients on levetiracetam exist. There is one case report showing a patient with atrial fibrillation, essential thrombocythemia, and epilepsy. He had recurrent transitory ischemic attacks during levetiracetam treatment and immeasurable trough rivaroxaban levels on one occasion. Two months after levetiracetam was changed to lacosamide his trough plasma rivaroxaban levels were in the expected range and transitory ischemic attacks had stopped [16].
A recent study on an Asian population also reports no recurrent thromboembolic events in a small group of post-stroke atrial fibrillation patients receiving levetiracetam and NOACs during a median of 30 months of follow-up [17]. On the contrary, a cohort study from a health service database in Israel included 79,302 patients with atrial fibrillation with newly dispensed NOAC showed an increased risk for thromboembolic events in those with concurrent prescriptions of antiepileptic drugs [18]. Giustozzi et al. also reported a high rate of ischemic stroke (5.6% patient-year) in atrial fibrillation patients treated with NOACs and different antiepileptic drugs [19]. Measurement of plasma Anti-factor Xa assay concentration should be recommended in all patients taking potentially interacting drugs, especially in situations of thromboembolic or bleeding event.
Conclusion
First, we suggest that future studies should include populations with clear and comparable baseline thrombotic risk, plan a sufficiently long follow-up, and consider possible interruptions/resumptions of anticoagulant and anticonvulsant treatments as time-dependent variables. Moreover, many patients receive other drugs in addition to AEs that also interact with NOACs, limiting the ability to attribute any adverse clinical outcomes to the NOACs-Anti epileptics Drug Interactions specifically and must be accounted for 25.
In conclusion, there are several potential DDIs between NOACs specifically between factor Xa inhibitors and AE drugs, but current evidence is limited and statistically underpowered to draw definitive conclusions regarding clinical significance. Unlike other AE drugs, Levetiracetam has no significant adverse effects and DDIs. When a possible pharmacological interaction between NOAC and AE is suspected in a group of individuals with recurrent bleeding or thromboembolic events, the VKORC1 inhibitor Warfarin can be considered an effective anticoagulant. Further studies with larger sample sizes, different ethnicities, direct monitoring of NOAC serum concentration, and longer follow-up duration or meta-analysis are warranted to clarify the clinical significance of DDIs between NOAC and AE drugs.
Declarations
Consent
Informed Consent was obtained.
Funding
Financial support and sponsorship – NIL.
Conflict of Interest
None.
Acknowledgments
The authors wish to thank AJ Hospital and Research Centre, Mangalore.
References
- Witt DM, Nieuwlaat R, Clark NP, et al. (2018). American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2:3257-3291.
Publisher | Google Scholor - Galgani A, Palleria C, Iannone LF, et al. (2018). Pharmacokinetic interactions of clinical interest between direct oral anticoagulants and antiepileptic drugs. Front Neurol. 9:1067.
Publisher | Google Scholor - Moerman L, Wyffels L, Slaets D, et al. (2011). Antiepileptic drugs modulate P-glycoproteins in the brain: a mice study with (11) C-desmethyl loperamide. Epilepsy Res. 94:18-25.
Publisher | Google Scholor - Mathy FX, Dohin E, Bonfitto F, et al. (2019). Drug-drug interaction between levetiracetam and non-vitamin K antagonist anticoagulants. Eur Heart J. 40:1571.
Publisher | Google Scholor - Mathy FX, Dohin E, Bonfitto F, Pelgrims B. (2019). Drug-drug interaction between levetiracetam and non-vitamin K antagonist anticoagulants. Eur. Heart J. 40(19):1571.
Publisher | Google Scholor - von Oertzen TJ, Trinka E, Bornstein NM. (2019). Levetiracetam and non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation and epilepsy: a reasonable combination. Eur. Heart J. 40(19):3800–3801.
Publisher | Google Scholor - Abou-Khalil B. (2008). Levetiracetam in the treatment of epilepsy. Neuropsychiatr. Dis. Treat. 4(3):507–523.
Publisher | Google Scholor - Steinhoff BJ, Staack AM. (2019). Levetiracetam and brivaracetam: a review of evidence from clinical trials and clinical experience. Ther. Adv. Neurol. Disord. 12:1756286419873518.
Publisher | Google Scholor - Nicolas JM, Collart P, Gerin B, Mather G, Trager W, et al. (1999). In vitro evaluation of potential drug interactions with levetiracetam, a new antiepileptic agent. Drug Metab. Dispos. 27(2):250–254.
Publisher | Google Scholor - Hole K, Wollmann BM, Nguyen C, Haslemo T, Molden E. (2018). Comparison of CYP3A4-inducing capacity of enzyme-inducing antiepileptic drugs using 4bhydroxycholesterol as a biomarker. Ther. Drug Monit. 40(4):463–468.
Publisher | Google Scholor - Zhang C, Kwan P, Zuo Z, Baum L. (2012). The transport of antiepileptic drugs by P-glycoprotein. Adv. Drug Deliv. Rev. 64(10):930–942.
Publisher | Google Scholor - Moerman L, Wyffels L, Slaets D, Raedt R, Boon P, et al. (2011). Antiepileptic drugs modulate P-glycoproteins in the brain: a mice study with (11) C-desmethyl loperamide. Epilepsy Res. 94(1–2):18–25.
Publisher | Google Scholor - Baltes S, Gastens AM, Fedrowitz M, Potschka H, Kaever V, et al. (2007). Differences in the transport of the antiepileptic drugs phenytoin, levetiracetam and carbamazepine by human and mouse P-glycoprotein. Neuropharmacology. 52(2):333–346.
Publisher | Google Scholor - Levy RH, Ragueneau-Majlessi I, Baltes E. (2001). Repeated administration of the novel antiepileptic agent levetiracetam does not alter digoxin pharmacokinetics and pharmacodynamics in healthy volunteers. Epilepsy Res. 46(2):93–99.
Publisher | Google Scholor - Grewal GK, Kukal S, Kanojia N, Madan K, Saso L, et al. (2017). In vitro assessment of the effect of antiepileptic drugs on expression and function of ABC Transporters and their interactions with ABCC2. Molecules. 22(10):1484.
Publisher | Google Scholor - Paciullo F, Costa C, Gresele P. (2020). Rivaroxaban plasma levels and levetiracetam: a case report [Letter] Ann. Intern. Med. 173(1):71–72.
Publisher | Google Scholor - Ho CJ, Chen SH, Lin CH, Lu YT, Hsu CW, et al. (2021). Non-vitamin K oral anticoagulants and anti-seizure medications: a retrospective cohort study. Front. Neurol. 11:588053.
Publisher | Google Scholor - Gronich N, Stein N, Muszkat M. (2021). Association between use of pharmacokinetic-interacting drugs and effectiveness and safety of direct acting oral anticoagulants: nested case–control study. Clin. Pharmacol. Ther. 110(6):1526–1536.
Publisher | Google Scholor - Giustozzi M, Mazzetti M, Paciaroni M, Agnelli G, Becattini C, et al. (2021). concomitant use of direct oral anticoagulants and antiepileptic drugs: a prospective cohort study in patients with atrial fibrillation. Clin. Drug Investig. 41(1):43–51.
Publisher | Google Scholor