Research Article
Diagnostic Methods and Outcomes of Clostridial Necrotizing Enterocolitis in Preterm Infants: A Retrospective Study
- Hanady Al Haddad *
- Suzanne Borrhomee
Neonatology department, Rene Dubos Hospital, France.
*Corresponding Author: Hanady Al Haddad, Neonatology department, Rene Dubos Hospital, France.
Citation: Hanady A. Haddad, Borrhomee S. (2024). Diagnostic Methods and Outcomes of Clostridial Necrotizing Enterocolitis in Preterm Infants: A Retrospective Study. Clinical Case Reports and Studies, BioRes Scientia Publishers. 5(2):1-10. DOI: 10.59657/2837-2565.brs.24.106
Copyright: © 2024 Hanady Al Haddad, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: January 30, 2024 | Accepted: February 14, 2024 | Published: February 26, 2024
Abstract
Background: Necrotizing enterocolitis (NEC) is the most severe and life-threatening gastrointestinal disease among preterm infants.
Objective: We evaluated the diagnostic methods for clostridial NEC in our center. We described the affected population, the feeding strategies used before the onset of NEC, the clinical outcomes of patients with clostridial NEC and the outbreak of clostridial NEC in our center.
Design: We performed a retrospective monocentric study in Rene Dubos Hospital in France. Our population included neonates with definite NEC corresponding to Modified Bell’s stages II, III, and IV associated with clostridium butyricum and neonatale, diagnosed between January 2017 and November 2022.
Results: Among the 7 neonates diagnosed with clostridium infection associated with NEC, 85% of our populations were exclusively fed with human milk. Slower and intermediate rates of progression of enteral feeding strategies were associated with a higher risk of NEC. Bacteriological diagnoses were announced positive by blood culture after 18 +/- 6 hours with unidentified anaerobic germ. Microbiota analysis showed that 71% of our populations were clostridium neonatale and 29% were clostridium butyricum. Surgical intervention was necessary for all patients. An outbreak of necrotizing enterocolitis occurred within a 1-month period, in 3 neonates of our population. Blood culture from these neonates grew the same strain, clostridium neonatal.
Conclusions: A slow rate of progression of enteral feeding is associated with an increased risk of developing NEC. Medical teams should provide aggressive therapeutic management, incorporate anaerobic BC into diagnostic workups, and consider the use of PCR test for early detection of clostridial infections. Outbreak of necrotizing enterocolitis associated with clostridium neonatal is common, appropriate broad-spectrum disinfectant with hand hygiene and quarantine rules should be applied.
Keywords: necrotizing enterocolitis; neonatal; intubation
Introduction
Necrotizing enterocolitis (NEC) is the most severe and life-threatening gastrointestinal disease among preterm infants that continues to account for substantial morbidity and mortality in neonatal intensive care units [1]. The major complications following NEC are the development of short bowel syndrome, intestinal stenosis, need for colostomy, and difficulty in reestablishing the early enteral diet [2]. The pathogenesis is complex and multifactorial. Prematurity, enteral feeding strategies, gut bacterial colonization, and inappropriate proinflammatory response are major factors implicated in NEC development [3]. Several causative microorganisms including viruses, gram-negative bacilli, and Clostridium spp have been proposed [5]. The most common clostridia species involved are C. butyricum and C. perfringens [6]. Recently, a novel Clostridium species, C. neonatale, was associated with a NEC outbreak [7]. All of these bacteria belong to the endogenous intestinal microflora, and the aberrant gut colonization observed in preterm infants may be a risk factor [8]. In this retrospective study, we describe the management of clostridium infection associated with necrotizing enterocolitis in our neonatal intensive care unit, highlighting the utility of early diagnosis and the use of early PCR detection for clostridium species.
Methodology
Study design: A retrospective monocentric study in Rene Dubos Hospital in France was conducted. We enrolled all neonates with definite NEC corresponding to Modified Bell’s stages II, III, and IV associated with clostridium butyricum and neonatale, diagnosed between January 2017 and November 2022. Infants who met criteria for NEC without associated clostridium species were not included. None of the selected patients had received probiotics.
Ethics: All methods were performed in accordance with the relevant guidelines. Data base was declared to the National Commission for Computing and Liberties (CNIL). Written information was delivered to all parents, ensuring their non-opposition to the research.
Outcomes: The primary outcome was to describe the diagnostic methods for clostridial NEC in our center. Additional outcome included describing the affected population, observing feeding strategies used before the onset of NEC, and describing outcomes of clostridial NEC in our center.
Data collection: recorded variables included gestational age, birth weight, IUGR, gender, mode of delivery, antenatal corticosteroids, postnatal antibiotics, APGAR score, speed of feeding, type of milk, tracheal intubation, hemodynamic failure suggested by clinical signs and /or abnormal laboratory results.
Statistics
The discrete data were presented as a percentage. Numerical data were presented by their median & quartiles. The statistical analysis was carried out with the R software & Excel.
Results
Population
In our unit, 7 neonates were diagnosed with a clostridium infection associated to NEC between 2017 and 2022. NEC was diagnosed based on clinical and radiological findings, classified using Modified Bell’s staging criteria. Patient’s characteristics are summarized in table1. 85% of our population was exclusively fed with human milk. The only patient who received formula was a 2020g neonate, born at 32+4 weeks.
Table 1: Characteristics of the population
| Characteristics of the population | |
| Gestationnal age (SA) | 29. 6 +/- 2 |
| Birth weight (g) | 1231 +/- 377 |
| IUGR < 10e> | 2 (28%) |
| Percentile | 48 +/- 36 |
| Male gender (n, %) | 1 (14%) |
| C-section (n, %) | 4 (57%) |
| Maternal antibiotics (n, %) | 5 (71%) |
| Initial antibiotics (n, %) | 3 (43%) |
| APGAR | |
| 1 | 7 |
| 5 | 9 |
| 10 | 9 |
| Complete prenatal steroids (n, %) | 6 (86%) |
| Day of 1st feeding | 1,1 +/- 0,3 |
| Speed of feedings (ml/kg/d) | 18 +/- 3 |
| Exclusive Human milk | 6 (85%) |
| death n, % | 1 (14%) |
Bacteriological diagnosis
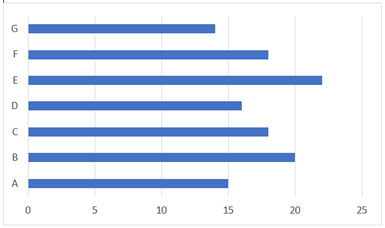
In all our patients, clostridium was found on a blood culture. Blood cultures were announced positive after 18 +/- 6 hours with unidentified anaerobic germ. Our laboratory used 16s rRNA gene sequencing which resulted in a long delay before precise identification of the clostridium species (Figure 1). Clostridium neonatal was the main species, present in 5 patients. When feasible, stool cultures were collected on the day of symptoms onset, but without specific search for clostridium species. Stool cultures came back negative. None of the selected patients had a positive clostridium species on milk culture samples.
Figure 1: Duration of bacteriological diagnosis (days)
NEC management and outcomes
Table 2: ECUN characteristics
| ECUN characteristics | |
| Day of NEC | 6,4 +/- 3 |
| Type of clostridium | |
| Neonatale | 5 (71%) |
| Butyricum | 2 (29%) |
| Initial rectal bleeding | 7 (100%) |
| Prolonged rectal bleding (>15j) | 2 (29%) |
| Hemodynamic instability | 7 (100%) |
| Need for surgery | 7 (100%) |
| Modified Bell’s stage | |
| III B | 5 (71%) |
| III A | 2 (29%) |
| CRP max | 128 +/- 46 |
| Lactate max | 7,2 +/- 0,13 |
| pH min | 6 +/- 5 |
| Quarantine* | 3 (43%) |
| Co infection | 3 (43%) |
| Associated germs | |
| Staph aureus | 1 (14%) |
| Staph, others | 2 (29%) |
| BGN | 1 (14%) |
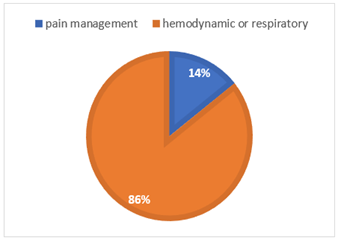
All patients presented a severe NEC with clinical sepsis (table 2). Hemodynamic instability was the main reason for intubation, before pain management (figure 2).
Figure 2: Cause for intubation
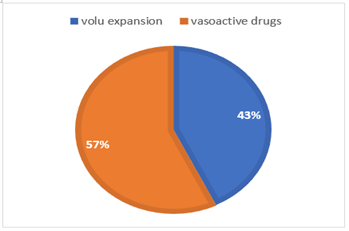
Vasoactive drugs were necessary in most patients (figure 3). The one patient who died was a 33 weeks neonate with 2nd percentile IUGR. He developed an early NEC at DOL 4. His condition deteriorated with a severe hemodynamic shock and a refractory hypoxia that failed to respond to resuscitation. He was infected with clostridium butyricum.
Figure 3: Hemodynamic management
All patients required surgery
5 patients required early surgery that consisted in a resection and protective colostomy. NPO after surgery lasted 7 +/- 0.5 days. Feeding was resumed with unfortified human milk, switched later on to extensively hydrolyzed formula if the mother did not supply enough breast milk. The duration between switching from total enteral feeding to premature baby formula was a one-month period. Reconstitution of intestinal continuity was performed after 8 +/- 0.3 weeks. For the remaining 2 patients who did not need early surgery, oral feeding was withheld for 7 and 9 weeks respectively. They experienced prolonged rectal bleeding, of 41 and 22 days. They both developed intestinal stenosis. The first patient, intestinal stenosis was confirmed by a barium enema at 7 weeks post enterocolitis. A resection of the small intestine stenosis and a direct anastomosis was organized within two weeks. Enteral feeding was resumed at day 3 post surgery. The second patient, intestinal stenosis was clinically diagnosed by a tender and bloated abdomen. Eventually, her clinical status deteriorated at 7 weeks post enterocolitis. She underwent an emergent surgery. A total colon stenosis with a 20 cm of small intestine necrosis was found. Protective enterostomy was performed and enteral feeding was resumed at day 7 post surgery.
Infection management
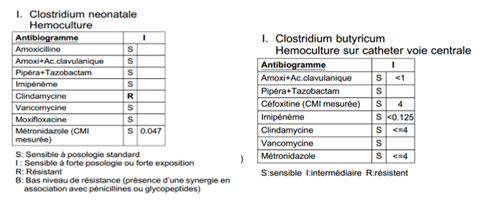
Broad spectrum antibiotics were started in 6 patients with cefotaxime, vancomycin, gentamicin and metronidazole. The one patient who died was the only patient who was initially treated with cefotaxime, gentamicin, vancomycin but without metronidazole. Due to severe hemodynamic shock, cefotaxime was switched to meropenem at day1 following NEC diagnosis. All clostridium had similar antibiogram (figure 4), and were sensitive to both flagyl and vancomycin. Initial biotherapy was continued in survivors for 18+/-3 days.
Figure 4
An outbreak of necrotizing enterocolitis occurred within a 1-month period, in 3 neonates of our population. Blood culture from these neonates grew the same strain, clostridium neonatal.
Those 3 were put in quarantine, with isolation and safety measures similar to the ones recommended for clostridium difficile.
Discussion
Population
There was a large majority of female neonates in our population (85.7%). There is no evidence of a gender specific sensitivity to clostridium infection in neonates. Patients did not necessarily present with known risk factors for NEC. The mean GA was over 28 weeks, only two of them were small for gestational age. Infants with clostridial NEC have more extensive pneumatosis intestinalis, gangrene and a more rapid progression of NEC than infants with non-clostridial NEC [6] All our patients needed rapid surgery either due to their extensive clinical deterioration, or later for an intestinal stenosis. One patient with clostridium butyricium eventually died.
Bacteriological diagnosis
Several causative microorganisms have been proposed in necrotizing enterocolitis. Nonetheless, the most implicated anaerobic bacteria from the commensal fecal microbiota are the family of clostridium [9] In 2014, based on a polyphasic study combining phylogenetic analysis and phenotypic characterization, Bouvet et al, clarified the status of C. neonatale, demonstrating that it is a new species belonging to cluster I of the Clostridium genus sensu stricto [10]. Particularly, this study permitted the differentiation of C. neonatale from another Clostridium species involved in NEC, Clostridium butyricum [11]. The differences between cases of NEC may be due to real differences in etiologies, or species but also to methodology. Application of molecular technique to the gut microbiota analysis showed that a large part cannot be cultivated under standard laboratory conditions. New methodologies are based on PCR techniques using universal primers or primers designed to amplify the variable 16S rRNA gene regions [12]. Van den Brand M et al introduced a newly developed multiplex PCR, as a rapid tool to enhance diagnosis of neonatal sepsis. (13) Direct clostridium species identification with PCR showed results available within 4 hours as compared with 14hours of incubation for blood culture which enables early initiation of targeted therapy. Further studies are needed to confirm that routine microbiological exams in preterm neonates with NEC should include bacterial culture under anaerobic conditions and RT-PCR testing specific for C.neonatale, C.butyricum and C. perfringens on a stool, milk, blood and surgical samples.
Antibiotics exposure
Cotten CM et al, demonstrated in their study that perinatal exposure to antibiotics has been identified as a risk factor for NEC, in particular when administered more than 5 days [15]. In our study, 43% of our population was exposed to perinatal antibiotics for less than 72 hours. Large spectrum antibiotics may have increased the risk of NEC. Moreover, the use of large spectrum antibiotics during the first episode of NEC may have participated by promoting gut dysbiosis to a secondary worsening episode.
Antibiotic treatment
Due to a lack of supportive evidence and guidelines, the length of treatment and choice of antimicrobial agents for presumed and proven episodes of NEC vary among centers. The study of Cantey et al. showed recently that the implementation of antibiotic stewardship programs including appropriate dosages and narrow-spectrum regimens was effective in reducing unnecessary antibiotic use in NICU [16]. 86% of our neonates received cefotaxime, vancomycin, gentamicin and metronidazole as a first treatment for NEC. The one patient who was clinically stable with low inflammatory marker did not receive metronidazole initially. He rapidly deteriorated and eventually died. Even though he had vancomycin, a bi-therapy could have been more effective against clostridium butyricum. We believe it is reasonable to always initiate metronidazole on the onset of NEC.
Nutrition
Though the etiology of NEC is multifactorial, several studies show that nutrition plays a major role as a preventive factor for necrotizing enterocolitis in neonates. Morgan J et al showed that advance enteral feeding volumes at daily increments of 30-35 ml/kg does not increase the risk of NEC in very preterm or very low birth weight infants. (17) Moreover, a delay in enteral feeding is associated with intestinal inflammation and a small volume of feeding has little or no impact on gut growth and maturation [18] However, in our center, the local protocol still recommended 20ml/kg daily increase, in order to prevent NEC. Thus, enteral feeding volume was increased at daily rate slightly under 20 ml/kg, resulting in several days delay in establishing full enteral feeding. Campos-Martinez AM et al, reviewed the protective effect of human milk on the inflammatory responses and provides immunological defenses to reduce the incidence of NEC (19). We maintained the human milk as only source of enteral feeding for our patients, since they were all below 32 weeks of GA or 1500g.
Prevention and Treatment
Various strategies have been proposed for the prevention of necrotizing enterocolitis. One of these preventing factors includes the routine use of different strains of probiotics, especially Lactobacillus and Bifidobacterium. However, the most suitable combination of strains and the optimum doses need further studies to be determined. (20-21) Therefore, probiotics are not routinely used in our NICU due to a lack of sufficient studies. Several studies isolate clostridium linked to outbreak cases of necrotizing enterocolitis. Dong Y et al showed an association between NEC and C. butyricum contamination within the hospital [24]. Also, Michelle J. Alfa et al, described an outbreak of necrotizing enterocolitis associated with clostridium neonatal within a 2-month period [25] 42.8 % of our population developed NEC in a period of 1 month at the same unit. Colonization in preterm neonates is highly influenced by iatrogenic manipulations including hospital environments. The ability of physicians and nurses to carry various clostridium species on their fingers if hand washing is not adequate has been suggested as a potential means of transmitting clostridium and this may be related to outbreak of NEC [25]. Spore forming bacteria are highly resistant to usual hospital disinfectants, so we used an appropriate broad-spectrum disinfectant, similar to the one used against clostridium difficile. Hand hygiene was performed with soap and water then alcohol-based hand rub. Patients were quarantined in single rooms.
Conclusion
Preterm infants with NEC may have a life-threatening evolution with extensive intestinal necrosis and high mortality. Medical teams should provide aggressive therapeutic management, incorporate anaerobic BC into diagnostic workups, and consider the use of PCR test for early detection of clostridial infections.
Knowledge about NEC due to Clostridium neonatal and butyricum has not been widely circulated, and given the significance of NEC, we decided to publish this study to alert the medical community to this ominous clinical presentation and facilitate diagnostic improvements.
Abbreviations
IUGR: intrauterine growth retardation
DOL: day of life
NICU: neonatal intensive care unit
IMV: invasive mechanical ventilation
PDA: patent ductus arteriosus
PRBC: packed red blood cells
CPAP: continuous positive airway pressure
IMV: invasive mechanical ventilation
NEC: necrotizing enterocolitis
EBM: expressed breast milk
Declarations
Ethics approval and consent to participate
This study was conducted in full compliance with ethical standards and guidelines. Written information was provided to all parents or guardians of the infants involved in the study, ensuring their understanding and non-opposition to the inclusion of their children’s data in the research.
Availability of Data and Material
Data supporting the finding of this study can be accessed from Rene Dubos Hospital’s archives.
Competing Interests
We have no competing interests.
Funding
This study did not receive any funding.
Acknowledgements
We would like to extend our heartfelt gratitude to all the patients and their families who participated in this case series. Their cooperation and patience were invaluable to the success of this study.
References
- C. Battersby, T. Santhalingam, K. Costeloe, N. Modi. (2018). Incidence of neonatal necrotizing enterocolitis in high-income countries: a systematic review, Archives of Disease in Childhood. Fetal and Neonatal Edition.
Publisher | Google Scholor - Thompson AM, Bizzarro MJ. (2008). Necrotizing Enterocolitis in Newborns. Drugs., 68:1227-1238.
Publisher | Google Scholor - Anand RJ, Leaphart CL, Mollen KP, Hackam DJ. (2007). The Role of The Intestinal Barrier in The Pathogenesis of Necrotizing Enterocolitis. Shock, 27:124-133.
Publisher | Google Scholor - Quigley M, McGuire W. (2014). Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev, 4:CD002971.
Publisher | Google Scholor - Kathleen Sim, Alexander G. Shaw, Paul Randell, et al. (2015). Dysbiosis anticipating necrotizing enterocolitis in very premature infants. Clin Infect Dis., 60:389-397.
Publisher | Google Scholor - Waligora-Dupriet A-J, Dugay A, Auzeil N, et al. (2005). Evidence for clostridial implication in necrotizing enterocolitis through bacterial fermentation in a gnotobiotic quail model. Pediatr Res., 58:629-635.
Publisher | Google Scholor - Alfa MJ, Robson D, Davi M, Bernard K, Van Caeseele P. (2002). Harding GK 2002 An outbreak of necrotizing enterocolitis associated with a novel Clostridium species in a neonatal intensive care unit. Clin Infect Dis, 35:S101-S105
Publisher | Google Scholor - Schwiertz A, Gruhl B, Lobnitz M, Michel P, Radke M, Blaut M. (2003). Development of the intestinal bacterial composition in hospitalized preterm infants in comparison with breast-fed, full-term infants. Pediatr Res, 54:393-399
Publisher | Google Scholor - L. Ferraris, M.J. Butel, F. Campeotto, M. Vodovar, J.C. Rozé, J. (2012). Aires, Clostridia in premature neonates’ gut: incidence, antibiotic susceptibility, and perinatal determinants influencing colonization. PLoS ONE, 7(2012):e30594.
Publisher | Google Scholor - Bouvet P, Ferraris L, Dauphin B, Popoff MR, Butel MJ, Aires J. (2014). 16S rRNA gene sequencing, multilocus sequence analysis, and mass spectrometry identification of the proposed new species Clostridium neonatale. J Clin Microbiol., 52:4129-4136.
Publisher | Google Scholor - Howard FM, Bradley JM, Flynn DM, Noone P, Szawatkowski M. (1977). Outbreak of necrotising enterocolitis caused by Clostridium butyricum, Lancet ii:1099-1102.
Publisher | Google Scholor - Schönherr-Hellec S, Aires J. (2019). Clostridia and necrotizing enterocolitis in preterm neonates. Anaerobe, 58:6-12.
Publisher | Google Scholor - Van den Brand M, Van den Dungen FA, Bos MP, Van Weissenbruch MM, Van Furth A, De Lange A, et al. (2018). Evaluation of a real-time PCR assay for detection and quantification of bacterial DNA directly in blood of preterm neonates with suspected late-onset sepsis. Critical Care, 22(1):1-08.
Publisher | Google Scholor - Makhoul IR, Smolkin T, Sujov P, Kassis I, Tamir A, Shalginov R, Sprecher H. (2005). PCR-based diagnosis of neonatal staphylococcal bacteremias. Journal of clinical microbiology, 43(9):4823-4825.
Publisher | Google Scholor - Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, et al. (2009). Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics, 123:58-66.
Publisher | Google Scholor - Cantey JB, Wozniak PS, Pruszynski JE, Sánchez PJ. (2016). Reducing unnecessary antibiotic use in the neonatal intensive care unit (SCOUT): a prospective interrupted time-series study. Lancet Infect Dis., 16:1178-1184.
Publisher | Google Scholor - Morgan J, Young L, McGuire W. (2014). Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev, 12:CD001241.
Publisher | Google Scholor - Konnikova Y, Zaman MM, Makda M, D’Onofrio D, Freedman SD, Martin CR. (2015). Late enteral feedings are associated with intestinal inflammation and adverse neonatal outcomes. PLoS One., 10:e0132924.
Publisher | Google Scholor - Campos-Martinez AM, Expósito-Herrera J, Gonzalez-Bolívar M, Fernández-Marin E, Uberos J. (2022). Evaluation of Risk and Preventive Factors for Necrotizing Enterocolitis in Premature Newborns. A Systematic Review of the Literature. Frontiers in Pediatrics, 10.
Publisher | Google Scholor - Cassir N, Benamar S, La Scola B. (2016). Clostridium butyricum: from beneficial to a new emerging pathogen. Clinical Microbiology and Infection, 1:22(1):37-45.
Publisher | Google Scholor - Thymann T, Møller HK, Stoll B, Støy ACF, Buddington RK, Bering SB, et al. (2009). Carbohydrate maldigestion induces necrotizing enterocolitis in preterm pigs. Am J Physiol Gastrointest Liver Physiol, 297:G1115-G1125.
Publisher | Google Scholor - Patel RM, Underwood MA. (2018). Probiotics and necrotizing enterocolitis. InSeminars in pediatric surgery, 27(1):39-46.
Publisher | Google Scholor - Liu Y, Tran DQ, Rhoads JM. (2018). Probiotics in disease prevention and treatment. The Journal of Clinical Pharmacology, 58:S164-S179.
Publisher | Google Scholor - Dong Y, Li Y, Zhang D, Nguyen S,Maheshwari N, Hu Y, et al. (2020). Epidemiological and genetic characterization of Clostridium butyricum cultured from neonatal cases of necrotizing enterocolitis in China. Infect Control Hosp Epidemiol, 41:900-907.
Publisher | Google Scholor - Alfa MJ, Kabani A, Lyerly D, Moncrief S, Neville LM, et al. (2000). Characterization of a toxin A-negative, toxin B-positive strain of Clostridium difficile responsible for a nosocomial outbreak of Clostridium difficile-associated diarrhea. Journal of clinical microbiology. 38(7):2706-2714.
Publisher | Google Scholor