Research Article
Diabetic Neuropathic Pain: Pathophysiology & Treatment
- Johannes B. Geeso *
- Cinthya I. Galan
Geisinger Commonwealth School of Medicine, USA.
*Corresponding Author: Johannes B. Geeso, Geisinger Commonwealth School of Medicine, USA.
Citation: Johannes B. Geeso, Cinthya I. Galan. (2023). Diabetic Neuropathic Pain: Pathophysiology & Treatment. International Clinical and Medical Case Reports, BRS Publishers. 2(2); DOI: 10.59657/2837-5998.brs.23.015
Copyright: © 2023 Johannes B. Geeso, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: May 18, 2023 | Accepted: May 30, 2023 | Published: June 05, 2023
Abstract
Diabetes mellitus is the global epidemic of the 21st century affecting millions worldwide. Usually, patients with long-standing type one diabetes (T1D) or type two diabetes (T2D) tend to develop complications. A common complication is the degenerative of the nerves in the peripheral nervous system (PNS). This complication is known as distal symmetric polyneuropathy or just diabetic neuropathy. A variety of symptoms can be experienced with this complication, including pain and numbness.
One characterization of this complication is the sensory loss that begins in the lower extremities and spreads substantially to the fingers and hand. There is no clear mechanism for how this happens in diabetic patients. Studies have shown that nerve damage from this complication can be due to oxidative stress, sorbitol accumulation, and advanced glycation end products (AGEs). Some of the most common medicationsbeing provided for diabetic neuropathic pain (DNP) include amitriptyline and desipramine. Other medications also include gabapentin and duloxetine. There are non-invasive options such as acupuncture that could provide beneficial data if rigorous studies are conducted. A number of new studies are being completed to identify more effectivetreatment for this condition.
Keywords: diabetics; neuropathic pain; pathophysiology; treatment
Introduction
A common and widespread health issue today is diabetes mellitus, commonly referred to as diabetes [1]. It is an epidemic known globally [1]. The lifestyle and behavior of humans have changed dramatically over the past century, resulting in an increase in diabetes worldwide [1]. Cardiovascular disease leads to about 18 million deaths, with diabetes and hypertension being prominent factors [2]. According to the Centers for Disease Control and Prevention, diabetes prevalence in the United States is expected to be close to 10% by 2020 and is increasing by about 5 percent each year [3]. It is predicted to double worldwide between 2000 and 2030 and reach pandemic levels by then [3]. Diabetic patients are estimated to incur a medical cost of $6,632 annually. The cost of medical expenses for people with DPN is double the cost of patients without DPN. In the event that DPN becomes severe, the patient will be forced to pay four-times as much as they did before, which comes out to $53,056[3].
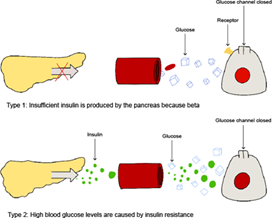
When it comes to diabetes, hyperglycemia, a condition characterized by excessive glucose levels in the blood, can be a defining characteristic. The disease can be categorized into two types: type one diabetes(T1D) or type two diabetes(T2D). T1D is the result of the autoimmune destruction of the beta cells (Fig. 1). T2D is found in patients with resistance to the action of insulin (Figure 1) [4].
Figure 1: The two different types of diabetic mellitus.
Complications are more likely to develop in patients with long-standing diabetes. It affects both T1D and T2D. The prevalence of complications is about 8% for newly diagnosed patients, and patients with long-standing diseases are at greater risk of developing it by more than 50% [5]. Among the complications are somatosensory disorders of the peripheral nervous system (PNS) [6]. This complication is also known as diabetic neuropathic pain (DNP). A variety of symptoms are associated with diabetic peripheral neuropathy (DPN), and a poor quality of life is often experienced. Nerve degeneration in the PNS is a clinical feature of DPN. Despite current pharmacotherapies treating symptoms and monitoring blood levels, diabetic patients will eventually develop this neurological condition [7]. DNP can be classified into four types:
distal symmetric polyneuropathy (DSPN), autonomic neuropathy, radiocomplexes neuropathy, and mononeuropathy. The most common type of DNP is the DSPN [8]. Approximately half of the diabetic patients are affected by this disease [9]. DNP is a common complication of diabetes, but the mechanism of how patients obtain this complication is elusive [10]. The following sections discuss the epidemiology, symptoms patients present, pathophysiology, and treatment options.
Epidemiology
Diabetic polyneuropathy pain is a common complication in the diabetic community affecting those with T1D and T2D. Despite the different patho-physiology fundamentals for T1D and T2D, the mechanism that leads to diabetic polyneuropathy has long been presumed to be the same [11]. About 95% of diabetics have type T2D, though the percentage of people who develop neuropathy is slightly lower-about 45%. Compared to T1D, the incidence of obtaining neuropathy is about 54% to 59% [3].
Hyper glycemia treatment in T1D significantly reduces the incidence of neuropathy by 60% to 70% [3]. In T2D, controlling glucose only reduces neuropathic pain by about 5% to 7%. Even though diabetic polyneuropathy occurs with long-standing hyper glycemia, even patients with reasonable glycemic control are at risk [3].
It is estimated that 75% of all diabetic neuropathies are caused by DSPN. The prevalence of T1D patients with DPN was only about 6% initially, but it increased to 30% as time passes [12]. Among patients with T2D, the baseline for DNP was 42%. Patients with T2D are already seven times greater for diabetic neuropathy than those with T1D. This complication tends to be a burden for older patients and adults with a long history of T1D or T2D [13]. About 50% of adults diagnosed with diabetes will be affected by this complication [14].
Patient Presentation
Diabetic neuropathy is a neurodegenerative disorder in the peripheral nervous system of diabetic patients [15]. Though it is a common complication for diabetic patients, it affects the individual quality of life [16]. As of today, no test can confirm whether a patient suffers from neuropathic pain, although a screening test can be used to determine whether they are suffering from DNP [17]. The screening involves Type II diabetic patients who are evaluated on diastolic blood pressure, creatinine serum levels, foot inspection, and risk factors that include measurement of hemoglobin A1c, history of smoking, and alcohol abuse [15]. A provider can also assess by observing how individuals walk or if there is any burning, numbness, or aching at the feet. This screening is recommended to be yearly after the first five years from when a T1D patient was diagnosed with DNP. For any T2D patient screening is done yearly from the first diagnosis. These symptoms are presented usually in the patient's toes and spread proximally. Patients have reported the spreading-like stocking-glove pattern. Once it reaches knee level, the pain, tingling, and numbness moves to the fingers [18]. Patients presenting with more symptoms or signs of nerve dysfunction can be a strong indicator of the diagnosis. Another indicator to help diagnose DNP is monitoring glycated hemoglobin (HbA1c) levels. With this condition, there is no explanation for why certain diabetic patients develop the painful version of the disease while others do not [11].
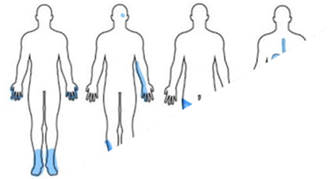
In diabetic patients, peripheral neuropathy is the most common complication. Approximately one-third to one-half of them have peripheral neuropathy. It is a nerve pain that affects the feet and legs and could also affect the hands and arms presented in Figure 2A [19].
Those with focal neuropathies have damage to single nerves, as shown in Figure 2B. These damages are often located in the hand, head, leg, and torso. Among the most common types of focal neuropathy pain is entrapment syndrome, which includes carpal tunnel syndrome. Any other types of focal neuropathy pain are not expected [19]. Since other focal neuropathies do not involve trapped nerves, they are much less common and often mainly affect older adults.
The proximal neuropathy is a rare condition affecting the hip, thigh, and buttock nerves shown in Figure 2C. The pain occurs only on one side of the body and does not spread to the other side of the body. The symptoms presented in proximal neuropathy progressively improve with time [19].
Autonomic neuropathy is obtaining damage to the nerves that control the internal organs. This damage can lead to problems with heart rate and blood pressure, the digestive system, bladder, sex organs, sweat glands, and eyes [19]. This type is represented in Figure 2D.
Figure 2: The four different types of diabetic neuropathy
Pathophysiology
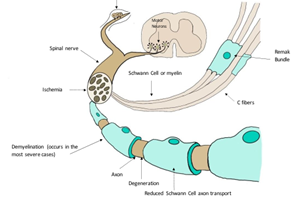
Although several mechanisms have been proposed as explanations for diabetic neuropathy, the mechanism remains elusive. The PNS relays messages between the central nervous system and the body. In diabetic neuropathy, the leading causes of peripheral nerve damage are oxidative stress, sorbitol accumulation, and advanced glycation end products (AGEs). In diabetic patients, different mechanisms are responsible for causing cellular damage. However, it is not clear what mechanism causes cellular damage first [20]. Although diabetic neuropathy produces alterations in the PNS, these alterations impact Schwann cell-axon transport, protein expression in dorsal root ganglions, demyelination, heat shock protein, and poly ADP-ribose polymerase (Fig.3) [15].
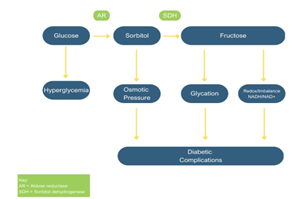
In diabetic neuropathy, the polyol pathway is one of the possibilities responsible for causing nerves to degenerate. The pathway is a two-step metabolic process that involves the conversion of glucose to sorbitol which then converts to fructose as the final product (Fig. 4) [21]. The mechanism of diabetic neuropathy can be viewed from this perspective in an attempt to understand the mechanisms involved. The enzymes involved in the mechanism are present in human tissues and organs. Diabetes complications are caused by these enzymes when intracellular glucose is elevated, leading to PNS alteration.
The level of sorbitol can increase when intracellular glucose levels are elevated, a condition known as hyperglycemia. Due to its hydrophilicity and alcoholic nature, sorbitol does not diffuse readily through cell membranes and builds up. Sorbitol accumulation can have several effects on the body [22]. Kidney function can be damaged during an accumulation of sorbitol, resulting in enzymuria and proximal tubular dysfunction [23]. Sorbitol accumulation also affects metabolic flux, which is the rate at which metabolites are turned over in the pathway [24]. The accumulation also affects Schwann cell function. In Figure 2, Schwann cells, also known for forming myelin, are present on the peripheral nerves. Peripheral nerves are covered with myelin, which allows messages between the central nervous system and the body to be relayed rapidly. Demyelinating peripheral nerves due to sorbitol accumulation will reduce the ability to relay messages between the central nervous system and the body [25]. A rat study showed that pain intensity decreased when the polyol pathway induced the accumulation of sorbitol and the Na+/K+-ATPase [26].
As a result of hyperglycemia, AGEs can also cause diabetic neuropathy. AGE modified peripheral nerve myelin is prone to phagocytosis by macrophages and contributes to the peripheral nerve's reduction [27]. Axonal degeneration and impaired axonal transport are the consequences of damage to the major components of the axonal cytoskeleton (tubulin, neurofilaments, and actin). A receptor for advanced glycation end products (RAGE) will bind to AGEs. This interaction will cause oxidative stress [27]. When proteins are under oxidative stress, their biological activity is decreased, which in turn results in a reduction in energy metabolism, cell signaling, transport, and cell death [28]. Glycoxidation products are produced more rapidly with the AGE and RAGE, inducing oxidative stress [27].
DRG
Figure 3: Sensory neurons
Figure 4: Diabetic complications
Treatments
Neuropathic pain is a condition that is not completely understood yet. Diagnosis and treatment of DPN can be assisted by some laboratory tests [29]. Due to this, there is still a large amount of trial and error to identify medications or treatments that work [30]. The US Food and Drug Administration has not approved any disease-modifying treatments for DPN [31]. The primary complication of diabetes is untreatable, so the symptoms of the disease are treated rather than the complication itself [32]. The class of drugs known as tricyclic antidepressants and selective serotonin reuptake inhibitors is the first line of therapy for DPN, which consists of drugs such as amitriptyline, pregabalin, gabapentin, and duloxetine [33]
Amitriptyline works by increasing noradrenergic and serotonergic neurotransmission due to the blocking of serotonin and norepinephrine transporters. One limitation of this medication presents is the potential side effects as it causes urinary retention, orthostatic hypotension, and sedation [32]. In order to view the result of amitriptyline a trial is needed to be conducted that includes a strict criterion for the patients. Glycemic levels needed to be under control with confirmation from the individual's primary care provider. Pain had to be consistent for a minimum of three months with pain that was no less than moderate. If there was any other medication being introduced into the body, it was interrupted three weeks in advance [32].
Patients were split into two groups: the same class of drugs, desipramine, and amitriptyline, were given to one group of participants. As a second group, participants were given fluoxetine, which blocks the reuptake transporter protein in the presynaptic terminal of serotonin neurons, and a placebo [34]. The study consisted of two six-week periods including a two-week washout period [34]. The range of doses was 12.5 to one hundred fifty mg per day for desipramine and amitriptyline. While twenty to forty mg per day were given of fluoxetine. The drugs were provided in a single dose at 9 PM, except in the patients who were non randomly assigned to the fluoxetine. A control group was included within this trial and these patients were instructed to take their medication at 9 AM. Instead of using sugar pills, the comparison group used benztropine. Benztropine selectively inhibits dopamine transporters but also binds to histamine and muscarinic receptors. A dose of 0.125 to 1.5 mg per day of benztropine was used to mimic the effects of dry mouth produced by desipramine and amitriptyline while causing no serious side effects [34].
Amitriptyline was given at an average dose of 105 mg, desipramine at an average dose of 111 mg, and fluoxetine at an average dose of 40 mg during the trial. There was a significant or moderate pain reduction in 74% of participants who received amitriptyline. The pain level of 61% of participants who received desipramine decreased significantly or moderately. A significant or moderate reduction in pain was experienced by 48% of participants who took fluoxetine. In the comparison group taking benztropine, there was a 41% reduction in significant or moderate pain. Desipramine's efficacy was similar to amitriptyline for DPN pain relief. Desipramine and amitriptyline were superior to the benztropine comparison group. A potential alternative to amitriptyline for the treatment of DPN is desipramine if the side effects of amitriptyline are too challenging for the patient to tolerate. In comparison with benztropine, fluoxetine had no greater effect on reducing patients' DPN. Another treatment that is also being used to treat DNP is Botulinum Toxin. Normally, this treatment is used to treat involuntary muscle movement and stiffness [35]. Most symptoms were improved by the end of a course of Botulinum [35]. The exact mechanism that alleviates neuropathic pain with this medication is BoNT hinders the secretion of pain mediators. Some of these mediators include substance P, glutamate, and calcitonin gene related protein from the nerve endings and dorsal root ganglions. This reduces local inflammation around the nerve endings, which inactivates the sodium channel and exhibits axonal transport [36]. To be more specific, hyperexcitability and spontaneous action potential mediated through the Na channel in peripheral sensory neurons contribute to the pathophysiology of neuropathic pain.
BoNT also controls neuropathic pain by blocking the Na channel [37]. During a randomized, double blind, placebo-controlled study Botulinum was confirmed to reduce pain after completing the study.
Although the mechanisms of its analgesics effect were not determined clearly, evidence from in vitro and in vivo experiments demonstrated the analgesic effect of BTX-A injection on neuropathic pain of diabetic patients [37]. A group of 57 Type II diabetic individuals were in a randomized double-blind placebo-controlled study. They all shared that they had neuropathic pain in both feet. They had injections placed in twelve different equally dispersed areas at the bottom of their feet. Pain levels were reported following a three-week period. The highest results were between two and fourteen weeks where pain was the lowest.
This study did not investigate long term effects, but it indicated positive results for future use of Botulinum Toxin. There was a decrease in the symptoms that were noted prior to the beginning of this study. Further studies should be done to determine the benefits and risk of using this medication for neuropathic pain [37].
Gabapentin, which blocks the tonic phase of nociception, is also a medication that is used to treat neuropathic pain [38]. In a group of one thousand individuals, three hundred eighty, after using gabapentin reported a minimum of a fifty percent pain decrease. Gabapentin given at a dose of 1800 to 3600 mg daily can produce good levels of pain relief in individuals with postherpetic neuralgia [38].
Acupuncture has been a method used as treatment for pain and DNP patients use it for pain relief. There was a significance in decreased pain, yet once the treatment was stopped, there was no long-term effect from treatment. The effects lasted a maximum of 16 weeks [39]. In addition, Acceptance and Commitment Therapy (ACT), one of the third-wave cognitive-behavioral therapies, was also performed. The goal of ACT is to confront, accept and change the patient's relationship to pain experience and psychological flexibility. [40] Ultimately this test had an outcome that ACT reduced pain to a high degree [40].
A new study conducted determined that intraneural facilitation (INF) treatment effectively restores blood flow to damaged nerves and relieves pain caused by DPN [41]. This was a hypothesis that was not tested before, yet it is known that reduced blood flow affects DPN. INF uses three grips or poses to dilate small openings in arteries surrounding nerves, improving blood flow to targeted nerves. Improved blood flow to the nerves stimulates healing and reduces or even stops neuralgia. It was possible to move forward with the possibility [41]. A single-blind trial assigned seventeen patients to receive INF and eleven patients to be in the control group. The group who received INF had this treatment given for sixty minutes three times a week for three consecutive weeks. The control group believed they were also receiving the same treatment for the same amount of time [41]. Both groups reported decreased pain following the study. Over time, decreased pain was significantly greater in the group that received INF [41].
In a spinal canal treatment, two small catheters or leads were inserted into the spinal canal, which advanced to the area where they were most effective with digital X- Ray imaging [42]. There are many patients who have experienced relief from this in some of the long-term studies they have conducted and have shown reductions of 75% to 80% in their symptoms [42]. Patients benefit from the treatment; however, High- frequency spinal cord stimulation (SCS) is only indicated for diabetes patients whose PDN has not responded to any other therapy [43] making it not easily accessible.
In every study, there are ways to improve it for future trials. DNP still has limited information available, which may be because the reasons for the failure of so many diabetic neuropathy treatments in randomized clinical trials are multifactorial. These include issues with trial design, participant selection, and endpoints, as well as the possibility that the therapy itself is ineffective [44]. An option that may help to relieve pain is to increase the patient's level of physical activity. In a small, randomized trial, NCS and vibration threshold improved by greater than 90% in patients with T1D and T2D after four years of exercise [45]. However, a larger trial must be conducted before accepting or rejecting the results.
To determine whether these medications that have had trials conducted are beneficial to patients suffering from diabetic neuropathy pain, there must be an increase in studies involving a broader patient population. Having a larger population will allow a more accurate representation of the population in today's society. Several adjustments need to be made, including the duration of the study and any other history the participant has. For example, other medications or a significant medical history that affect pain tolerance. There also needs to be an extended period for administering the medication. The purpose of this is to determine whether there are any adverse effects that may prevent the medication from being used. This is because it will not meet its intended purpose, or it will harm the patient. In the future, DPN will be treated preventatively and therapeutically [46].
Conclusion
There is a high prevalence of neuropathic pain among diabetics. Patients with this condition suffer from neurological disorders and their quality of life is adversely affected. Common symptoms are burning or numbness in the feet. This diagnosis can be classified into four types: autonomic neuropathy, focal neuropathy, proximal neuropathy, and peripheral neuropathy. The exact mechanism behind diabetic neuropathy is not known. A number of mechanisms, including the polyol pathway, have been proposed as possible causes. There is still a tremendous amount of research to be done to identify potential treatments for patients suffering from diabetic neuropathy. As a first-line treatment, desipramine and amitriptyline may be prescribed for these patients. Various options, such as Botulinum Toxin, SCS and acupuncture, will require more studies to confirm their effectiveness. As pathophysiology emphasizes disease, and treatment emphasizes symptoms, there is a limited correspondence between pathophysiology and treatment. In reference to previous studies mentioned there is a positive outcome, though larger studies are needed to represent to represent the entire population.
Acknowledgments
Thanks to Brian Piper, PhD for providing feedback on an earlier version, and Ambar Feliz for assisting in writing the references.
List of abbreviations
ACT: Acceptance and commitment therapy
AGE: Advance glycation end product
DNP: Diabetic neuropathic pain
DPN: Diabetic peripheral neuropathy
DPNP: Diabetic peripheral neuropathic pain
INF: Intraneural facilitation
NCS: Nerve conduction studies
PNS: Peripheral nervous system
RAGE: Receptor for advanced glycation end
SCS: Spinal cord stimulation
T1D: Type 1 diabetes
T2D: Type 2 diabetes
References
- .
-->